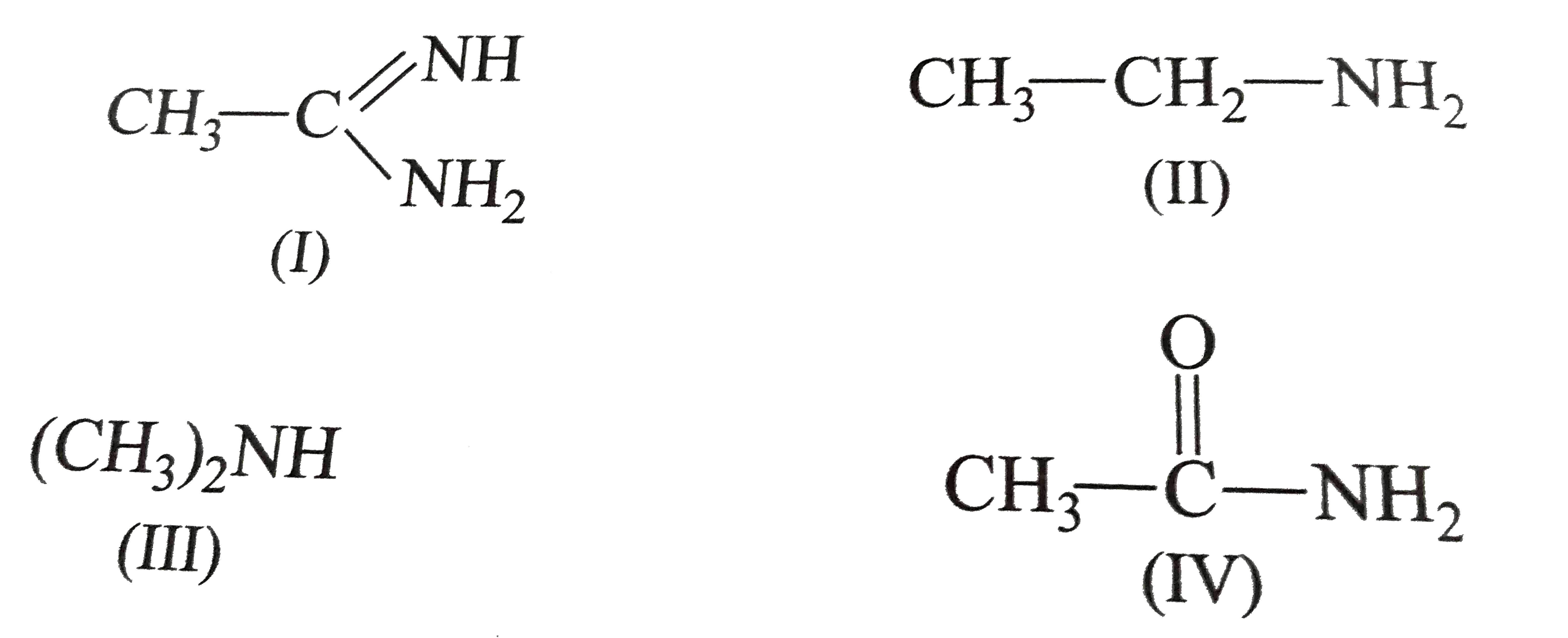A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Assertion-Reasoning Questions|7 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise AIPMT/NEET Questions|26 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section D - Chapter End Test|30 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING NITROGEN-AIIMS Questions
- Which of the following statement is true?
Text Solution
|
- The reaction is called
Text Solution
|
- Which of the following amines, can give N-nitrosoamine on treatment wi...
Text Solution
|
- The compound oxalate is used for distinguishing primary, secondary and...
Text Solution
|
- Diethyl oxalate is used for distinguishing primary, secondary and tert...
Text Solution
|
- Observe the following reaction: Which statement is not correct about...
Text Solution
|
- Which of the following reactions does not yield an amine?
Text Solution
|
- Urea upon hydrolysis yields
Text Solution
|
- Best method to form aromatic iodide is
Text Solution
|
- Benzamide and benzyl amine can be distinguished by
Text Solution
|
- The basic character of ethyl amine,diethyl amine and triethyl amine in...
Text Solution
|
- Which of the major product formed when C6H5CONHC6H5 undergoes nitratio...
Text Solution
|
- Nitrobenzene (PhNO2)overset(Zn+NH4Cl)toP, P will be
Text Solution
|
- Reaction of aniline with KNO2 followed by treatment of dilute acid giv...
Text Solution
|
- Which of the following will give carbylamine test?
Text Solution
|
- Which of the following does not give nitroalkane?
Text Solution
|
- What is the correct order of basicity among the following compounds?
Text Solution
|
- CH3-CH=CH-CH=N-CH3overset(LiAlH4)to What is final product
Text Solution
|
- What is sequence of reagent use to convert following
Text Solution
|
- Correct order of basic strength
Text Solution
|