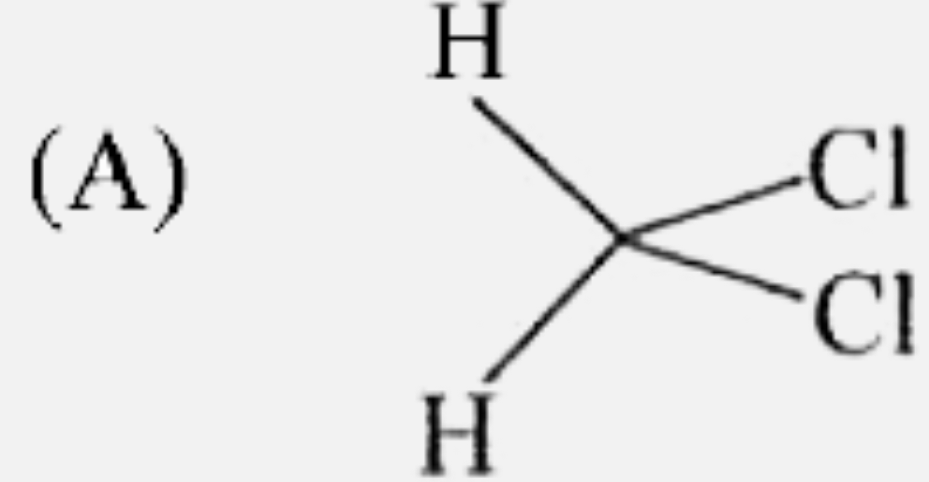
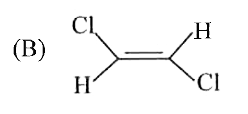
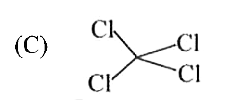
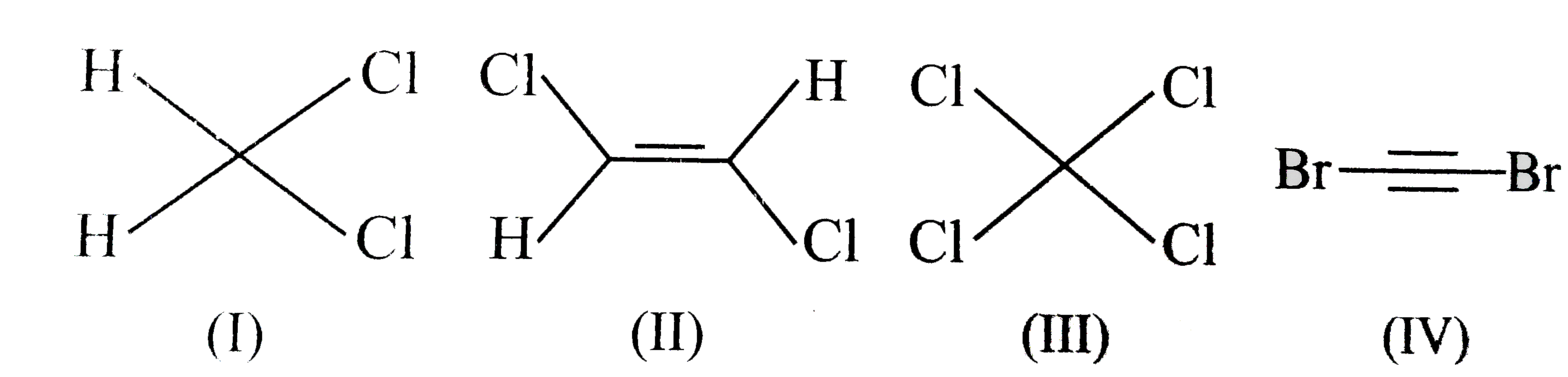A
B
C
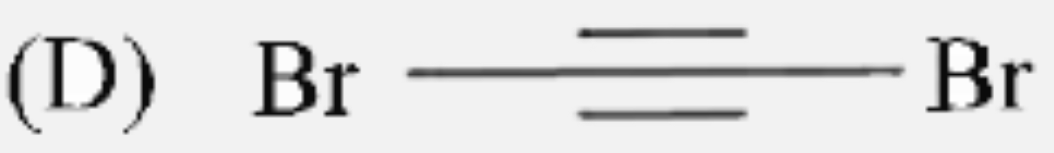
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-NATURE OF CHEMICAL BOND-Competitive Thinking
- Which molecules has zero dipole moment ?
Text Solution
|
- Write the structure of inorganic benzene ?
Text Solution
|
- The compound that will have a permanent dipole moment among the follow...
Text Solution
|
- polarisibility of halide ions increasing in the order
Text Solution
|
- Amongst LiCl, RbCl, BeCl2 and MgCl2, the compounds whith the greatrest...
Text Solution
|
- The correct sequence of increasing covalent character is represented b...
Text Solution
|
- Which of the following hybridisation is known as trigonal hybridisatio...
Text Solution
|
- In which molecule are all atoms co-planar?
Text Solution
|
- The species, having bonds angle of 120^(@) is
Text Solution
|
- Formation of PCl(3) is explained on the basis of what hybridisation of...
Text Solution
|
- The main axis of diatomic molecule is z. The orbitals px and py overla...
Text Solution
|
- Which type of overlapping reusults in the formation of a pi bond?
Text Solution
|
- The structure of PF(5) molecule is .
Text Solution
|
- The trigonal bipyramidal geometry results from the hybridisation
Text Solution
|
- Octahedral molecular shape exists in hybridisation.
Text Solution
|
- The structure of IF(7) is
Text Solution
|
- In a regular octahedral molecule MX(6) the number of X - M - X bonds ...
Text Solution
|
- The maximum number of 90^(@) angles between bond pair-bond pair of ele...
Text Solution
|
- Predicted the correct order among the following
Text Solution
|
- In the compound 'X', all the bond angles are exactly 109^(@)28. 'X' ma...
Text Solution
|