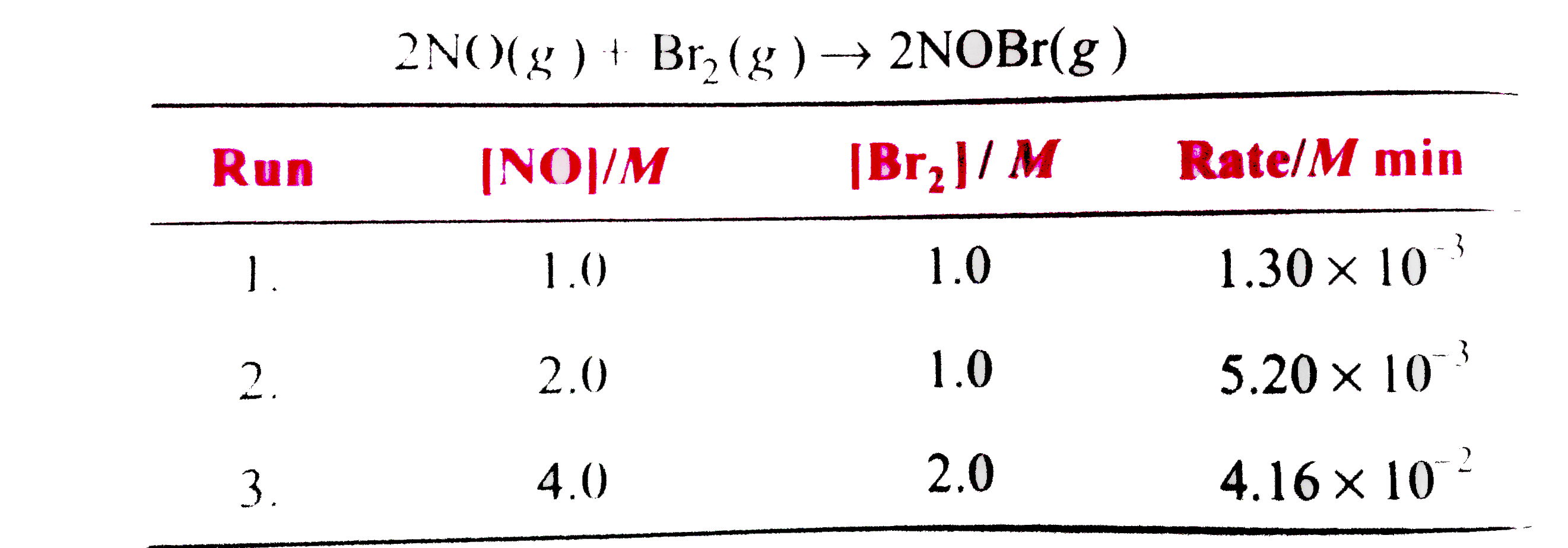Similar Questions
Explore conceptually related problems
Recommended Questions
- The following initial rate data were obtianed for the reaction 2NO...
Text Solution
|
- The reaction : 2N(2)O(5)(g) to 2NO(2)(g) + (O(2))(g) was studied and t...
Text Solution
|
- How can you determine the rate law of the following reactions? 2NO(g) ...
Text Solution
|
- The following initial rate data were obtianed for the reaction 2NO...
Text Solution
|
- For the reaction 2NO((g)) +Cl(2(g)) rarr 2NOCl((g)) the following data...
Text Solution
|
- The given data are for the reaction, 2NO(g)+Cl(g)rarr2NOCl(g)" at "298...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- For the reaction 2NO((g)) +Cl(2(g)) rarr 2NOCl((g)) the following data...
Text Solution
|
- How can you determine the rate law of the following reaction? 2NO((g))...
Text Solution
|