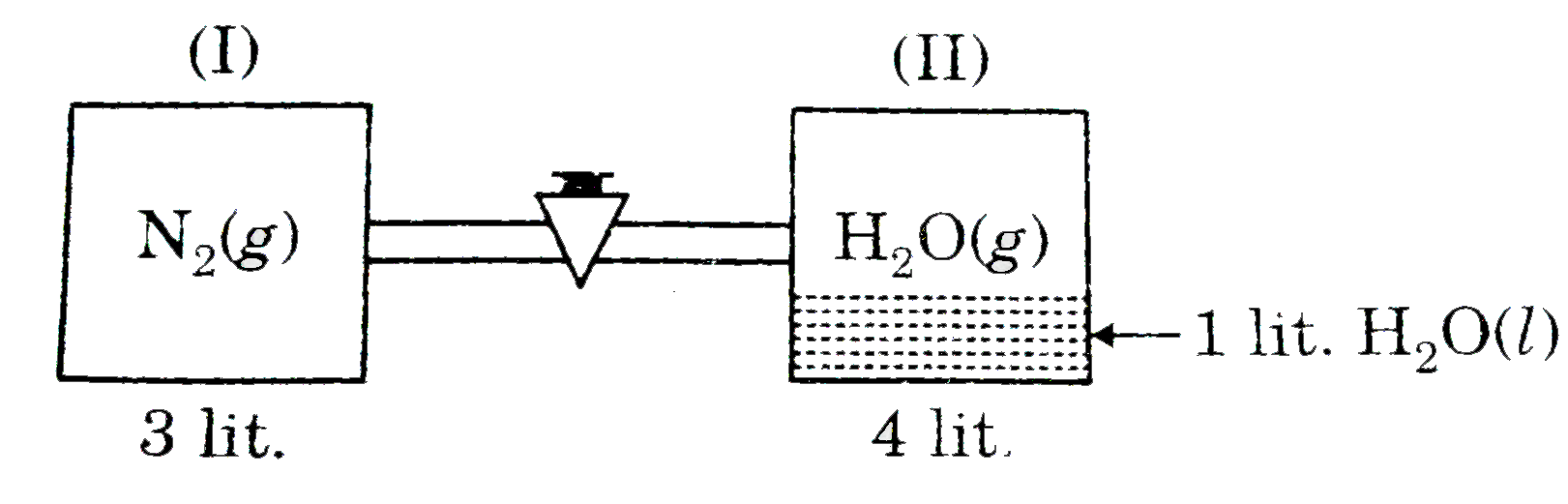Similar Questions
Explore conceptually related problems
Recommended Questions
- Two containers are connected by a tube of negligible volume container ...
Text Solution
|
- A 100 dm^(3) flask contains 10 mol each of N(2) and H(2) at 700 K . Af...
Text Solution
|
- Two containers are connected by a tube of negligible volume container ...
Text Solution
|
- A container is divided into two compartments. One compartment contains...
Text Solution
|
- Two adiabatic containers have volumes V(1) and V(2) respectively. The ...
Text Solution
|
- Two vessels connected by a valve of negligible volume. One container (...
Text Solution
|
- For reaction, assuming large volume of water. H(2)O(l)hArrH(2)O(g) ,...
Text Solution
|
- A container contains 32 g of O2 at a temperature T. The pressure of th...
Text Solution
|
- In a closed container of capacity V with N(2)(g) and wate vapour at 29...
Text Solution
|