Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following statements are correct regarding diborane? I....
Text Solution
|
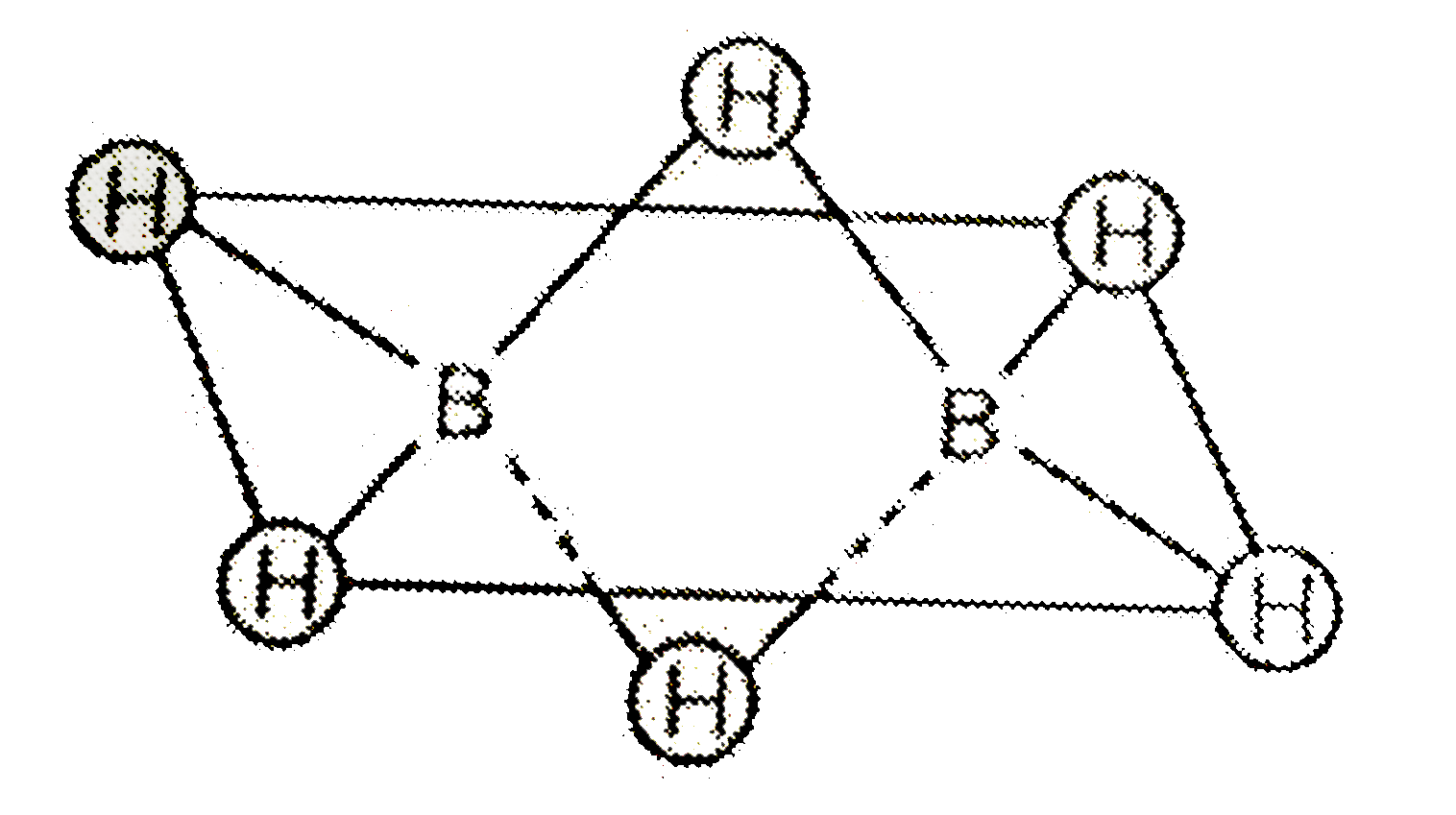
- The molecular shapes of diborane is shown below : . Consider the f...
Text Solution
|
- There are two H-bridge bonds in diborane molecule because there are
Text Solution
|
- Which of the following statements are correct regarding diborane? I....
Text Solution
|
- In diborane, the H-B-H bond angle is 120^(@). The hybridization of bor...
Text Solution
|
- Assertion : In diborane, each B atom is sp^(2) hybridised. Reason : In...
Text Solution
|
- Assertion : In diborane containing eight -B-H bonds, the four terminal...
Text Solution
|
- Assertion : In diborane containing eight -B-H bonds, the four terminal...
Text Solution
|
- Consider the following statements, (i) Diborane contains two centre tw...
Text Solution
|