Similar Questions
Explore conceptually related problems
Recommended Questions
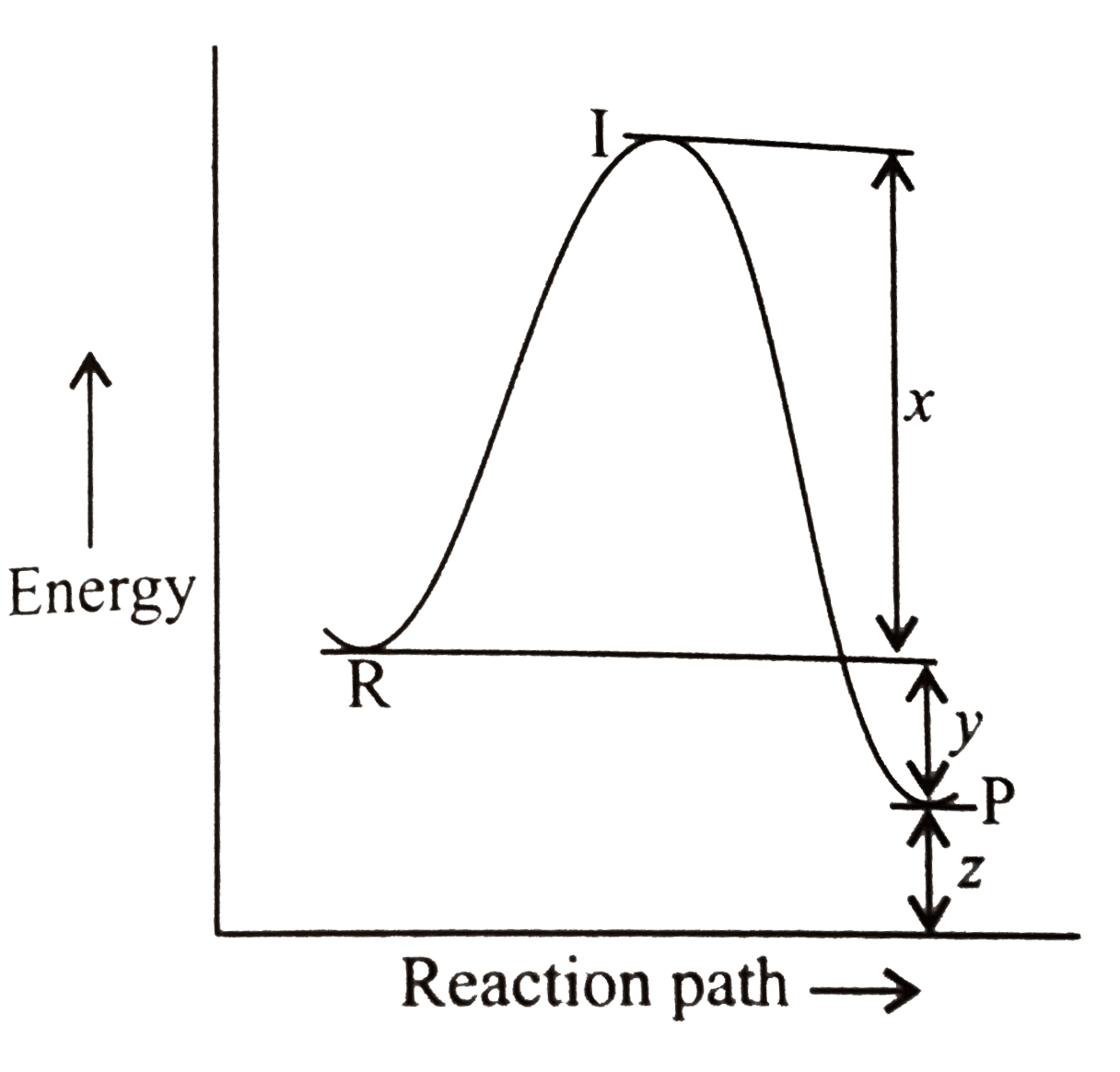
- The energy profile diagram for the reaction: CO(g)+NO(2)(g) hArr CO(...
Text Solution
|
- K(p)//K(c) for the reaction CO(g)+1/2 O(2)(g) hArr CO(2)(g) is
Text Solution
|
- The energy profile diagram for the reaction: CO(g)+NO(2)(g) hArr CO(...
Text Solution
|
- The energy profile diagram for the reaction: CO(g)+NO(2)(g) hArr CO(...
Text Solution
|
- The energy profile diagram for the reaction: CO(g)+NO(2)(g) hArr CO(2)...
Text Solution
|
- The energy profile diagram for the reaction: CO(g)+NO(2)(g) hArr CO(2)...
Text Solution
|
- For the reaction CO(g)+(1)/(2) O(2)(g) hArr CO(2)(g),K(p)//K(c) is
Text Solution
|
- For a reaction carried at 400 K, NO(2)(g) + CO(2)(g) to CO(2)(g) + NO(...
Text Solution
|
- K(p)//K(c) " for the reaction " CO(g) + 1/2 O(2) (g) hArr CO(2) (g) is
Text Solution
|