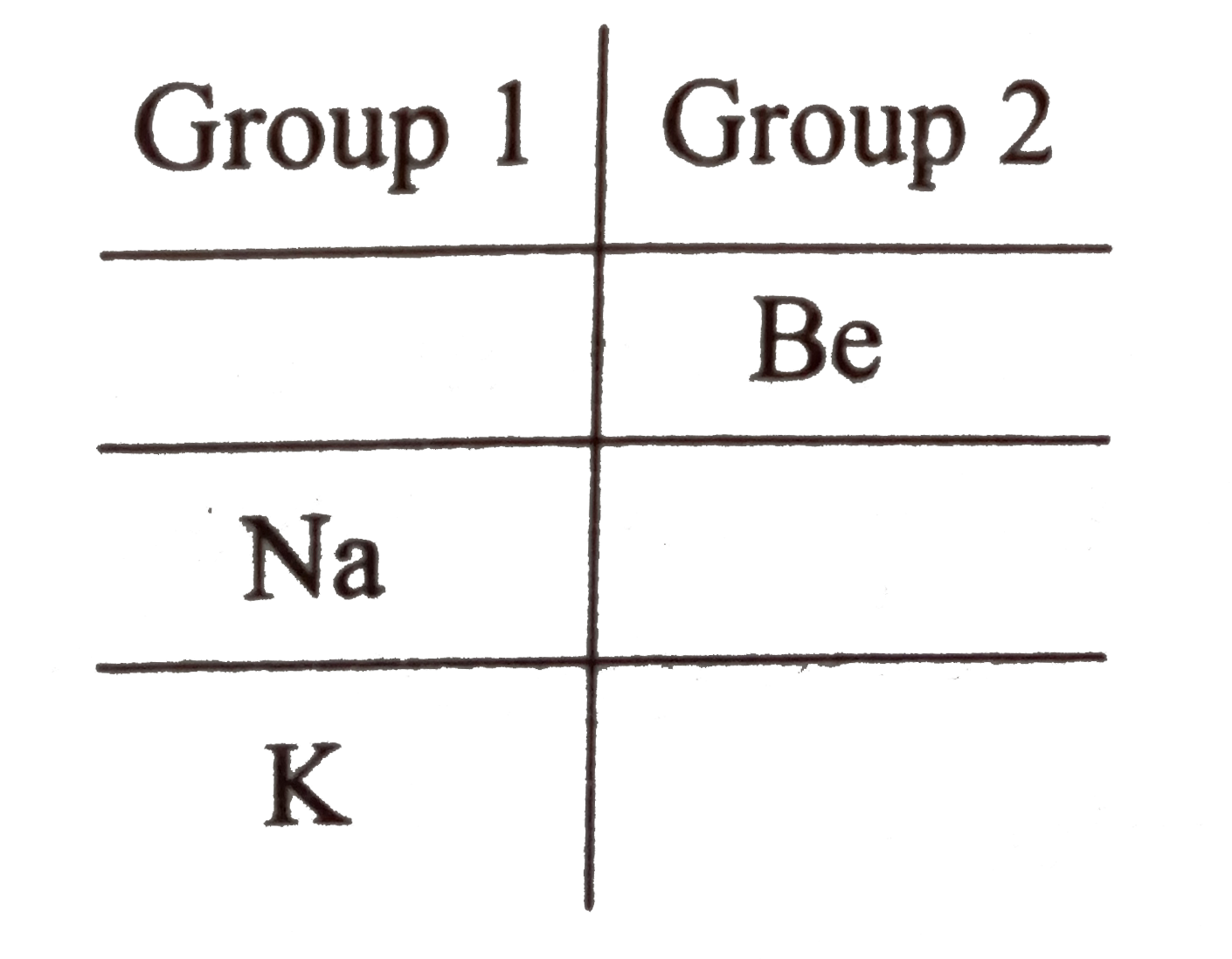A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise Level -II|1 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise Level -III|1 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise Level -I|1 VideosCHEMICAL BONDING
R SHARMA|Exercise Archives|73 VideosEQUILIBRIUM
R SHARMA|Exercise ARCHIVES|69 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS-Level
- An element X belongs to the fourth period and the fifteenth group of t...
Text Solution
|
- Which of the following two elements in the periodic table are expected...
Text Solution
|
- The correct order in which the first ionzation enthalpy increases is
Text Solution
|
- The least stable in amongst the following is :
Text Solution
|
- The lectronic configuration of the atom having maximum difference in f...
Text Solution
|
- The electronic configurations of four elements are given below. Arrang...
Text Solution
|
- The size of the following species increases in the order
Text Solution
|
- The successive ionzation enegry values for an element X are given belo...
Text Solution
|
- In which of the following arrangements, the order is according to the ...
Text Solution
|
- In a given shell, the order of screeing effect is
Text Solution
|
- Which one of the following sets of ions represents the collection of i...
Text Solution
|
- Consider the following statements: (i) The radius of an atom is lar...
Text Solution
|
- The first ionisation potential of Na is 5.1eV. The value of eectrons g...
Text Solution
|
- Which of the following represents the correct order of increasing firs...
Text Solution
|
- The increasing order of the first ionisation enthalpies of the element...
Text Solution
|
- The following statements regarding the periodic trends of chemical rea...
Text Solution
|
- Which of the following statements is not correct for the periodic clas...
Text Solution
|
- One mole of magnesium in the vapor state absored 1200 kJ mol^(-1) of e...
Text Solution
|
- In any period, the valency of an element with respect to oxygen
Text Solution
|
- Which of the following is correctly matched?
Text Solution
|