Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Archives
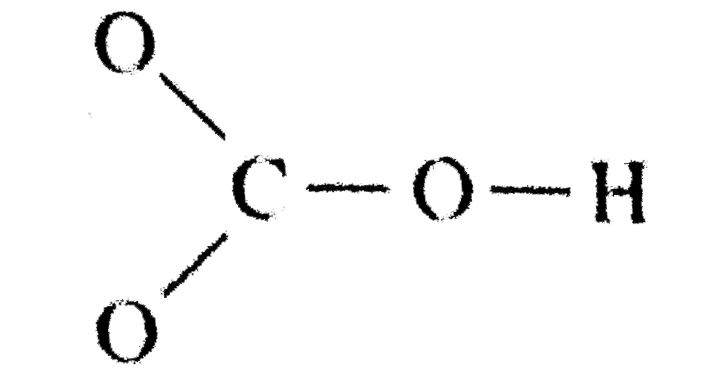
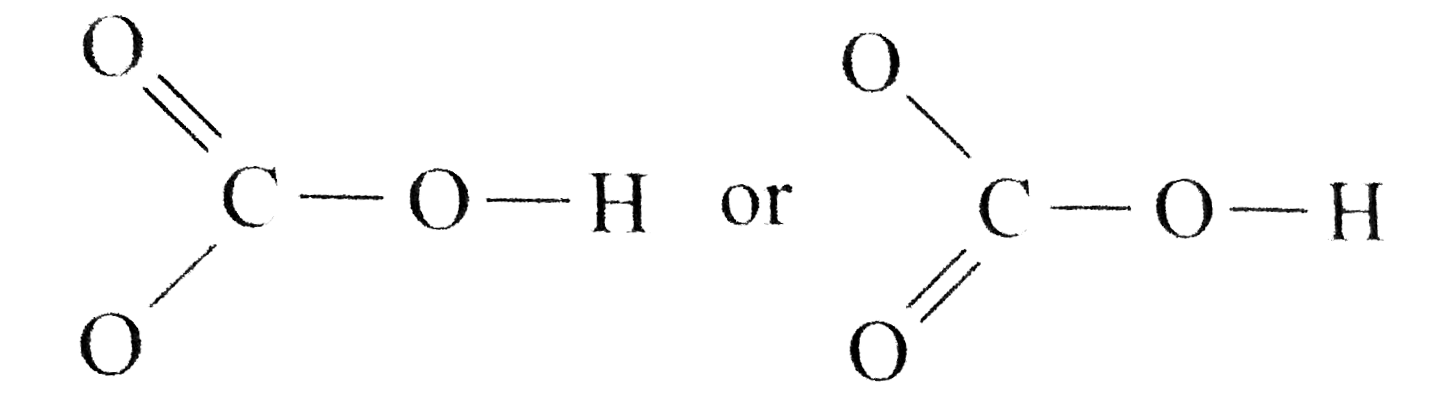
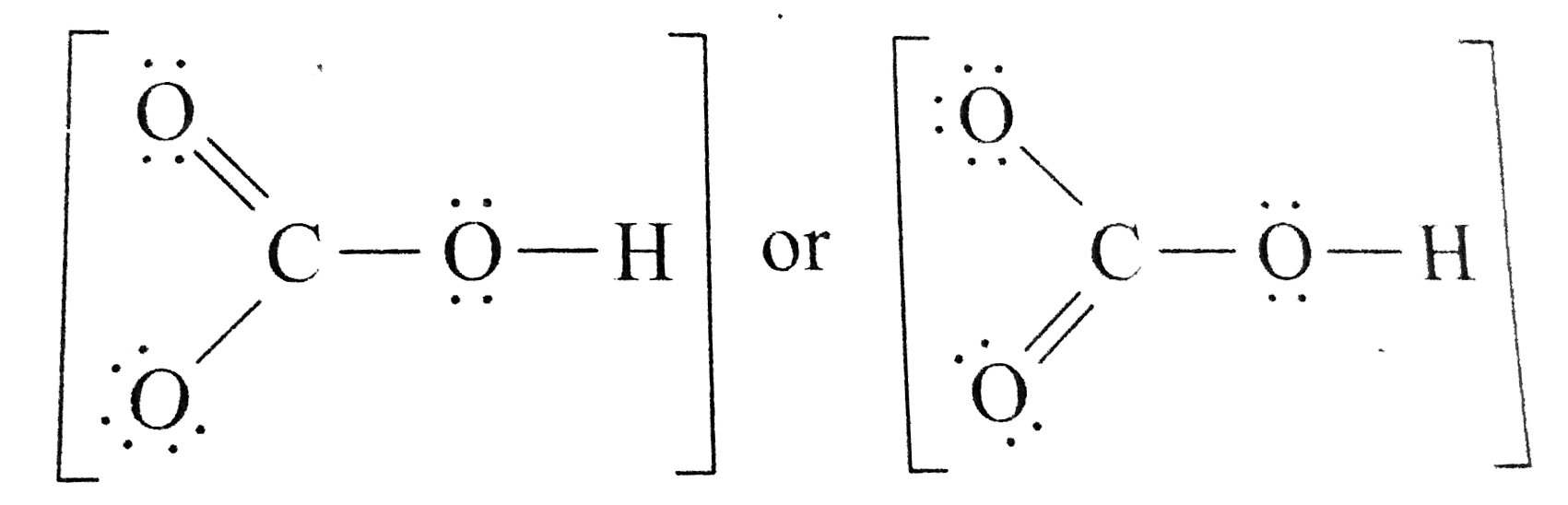
- Draw a Lewis structure for the bicarbonate ion, HCO3^(-).
Text Solution
|
- Which one of the following molecules contains no pi - bond ?
Text Solution
|
- Which of the following is a polar moleule ?
Text Solution
|
- Which of the following is paramagnetic?
Text Solution
|
- XeF2 is isostructural with
Text Solution
|
- In which of the following molecules/ions in the central atom sp^2-hybr...
Text Solution
|
- According to MO theory which of thhe following lists makes the nitroge...
Text Solution
|
- In the case of alkali metals, the covalent character decreases in the ...
Text Solution
|
- The state of hybridization of C2, C3, C5, and C6 of the hydrocarbon ...
Text Solution
|
- Arrange the following ions in the order of decreasing X-O bond length ...
Text Solution
|
- The enolic form of butanone contains
Text Solution
|
- Four diatomic species are listed in different sequence .Which of thes...
Text Solution
|
- The angular shape of none molecule (O(3)) consists of
Text Solution
|
- Which has the highest dipole moment?
Text Solution
|
- The hybridization of oxygen atom in H2O2 is
Text Solution
|
- Which one of the following pairs consists of only paramagnetic species
Text Solution
|
- The bond lengths and bond angles in the molecules of methane, ammonia,...
Text Solution
|
- The correct order of bond order values among the following (i) NO^(...
Text Solution
|
- A coordinate bond is a dative bond. Which of the following is true?
Text Solution
|
- In TeCl4, the central tellurium involves the hybridization
Text Solution
|
- In which of the following pairs are the two species isostructural?
Text Solution
|