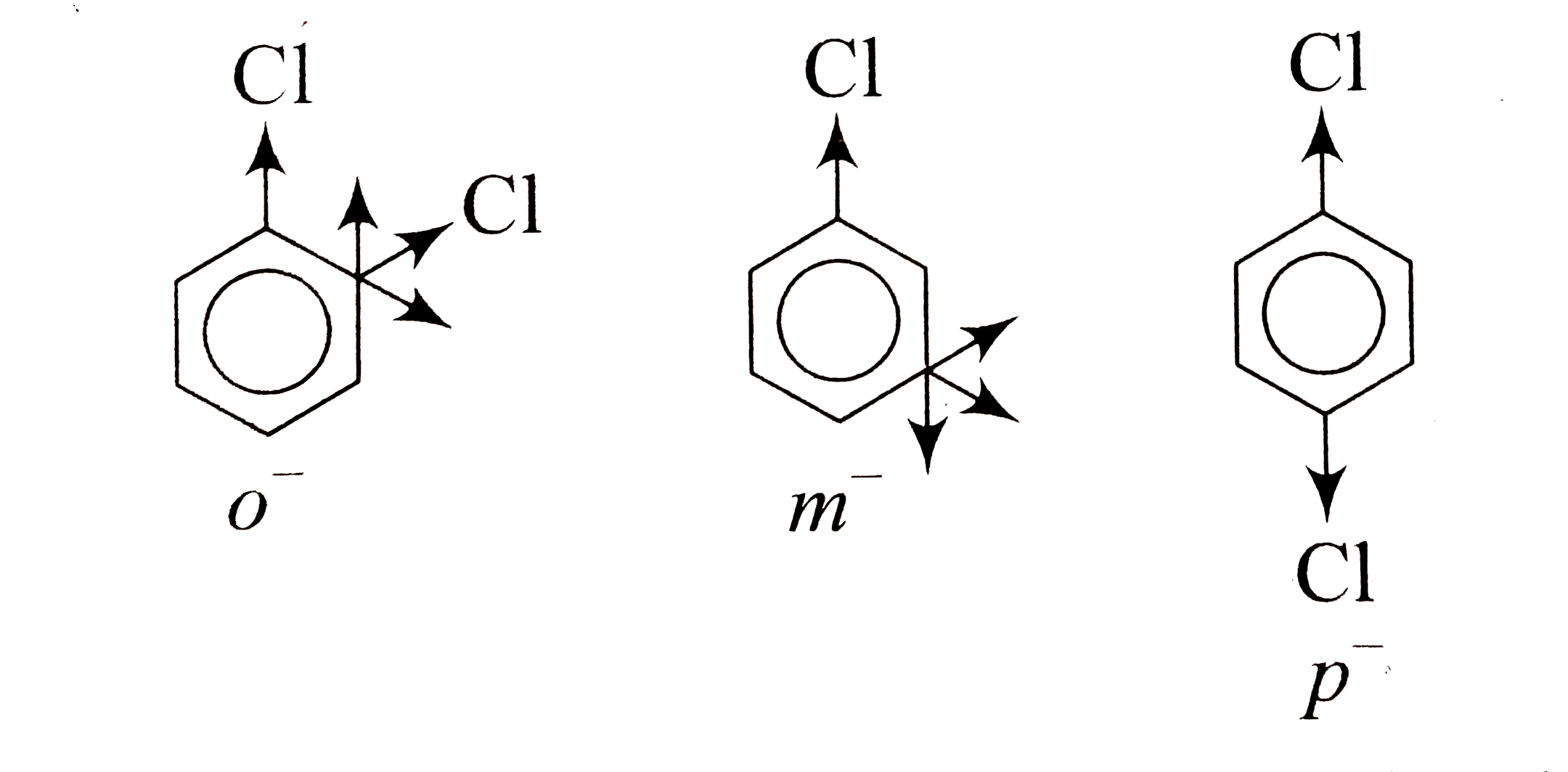A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 8|10 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 9|6 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 6|7 VideosAROMATIC HYDROCARBONS
R SHARMA|Exercise Archives|59 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Follow-up Test 7
- The bond between two identical nonmetal atoms has a pair of electrons
Text Solution
|
- Which contains both polar and non-polar bonds ? .
Text Solution
|
- Carbon tetrachloride has no net dipole moment because of
Text Solution
|
- Which of the following will have zero dipole moment?
Text Solution
|
- Which of the following molecule is nonpolar? (i) PbCl4 (ii) BF3 (i...
Text Solution
|
- The most polar bond is
Text Solution
|
- Which of the following has the highest dipole moment?
Text Solution
|
- Both CO2 and H2O contain polar covalent bonds but CO2 is nonpolar whil...
Text Solution
|
- Molecular size of ICI and Br2 is nearly same but b.pt. of ICI is abou...
Text Solution
|
- The observed dipole moment of HCl is 1.03D. If the bond length of HCL ...
Text Solution
|
- According to Fajan's rules, the maximum ionic character is favored by
Text Solution
|
- Which of the following has the highest covalent character?
Text Solution
|