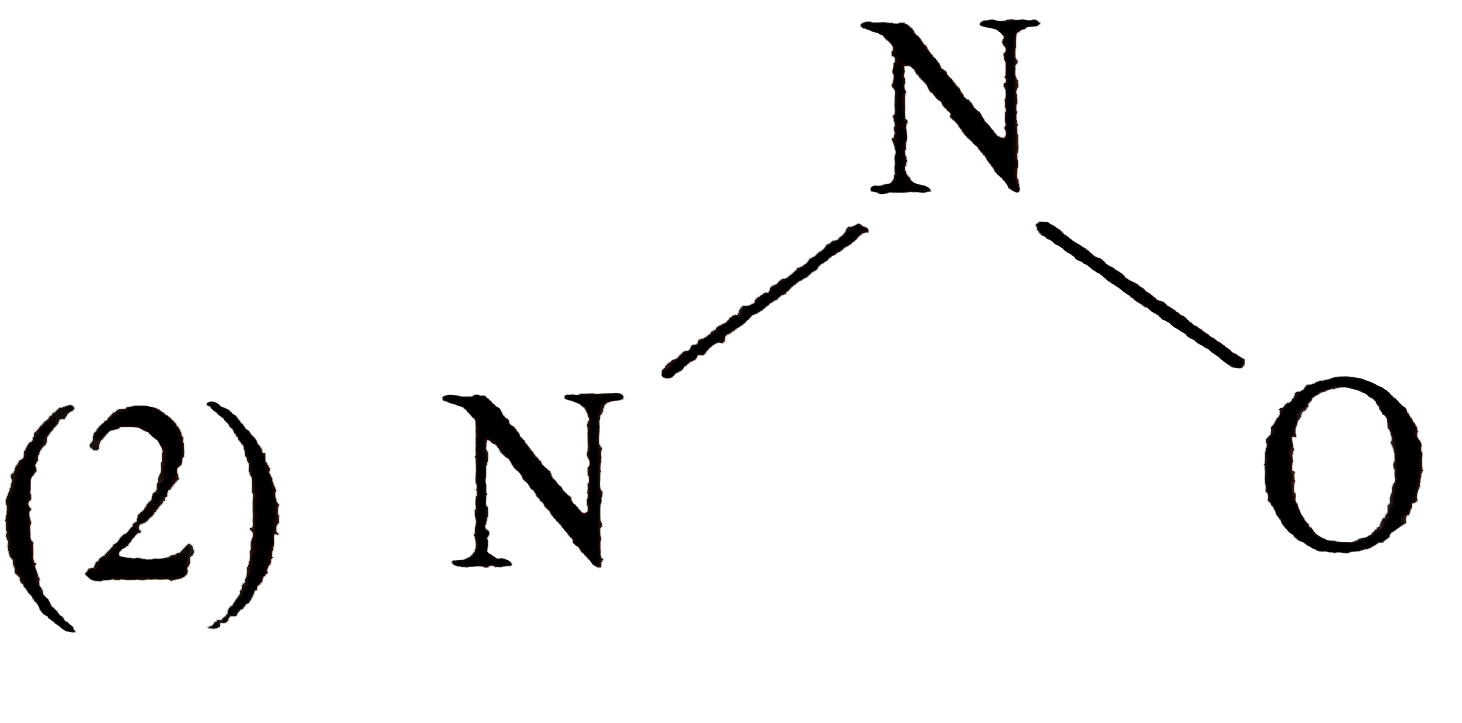
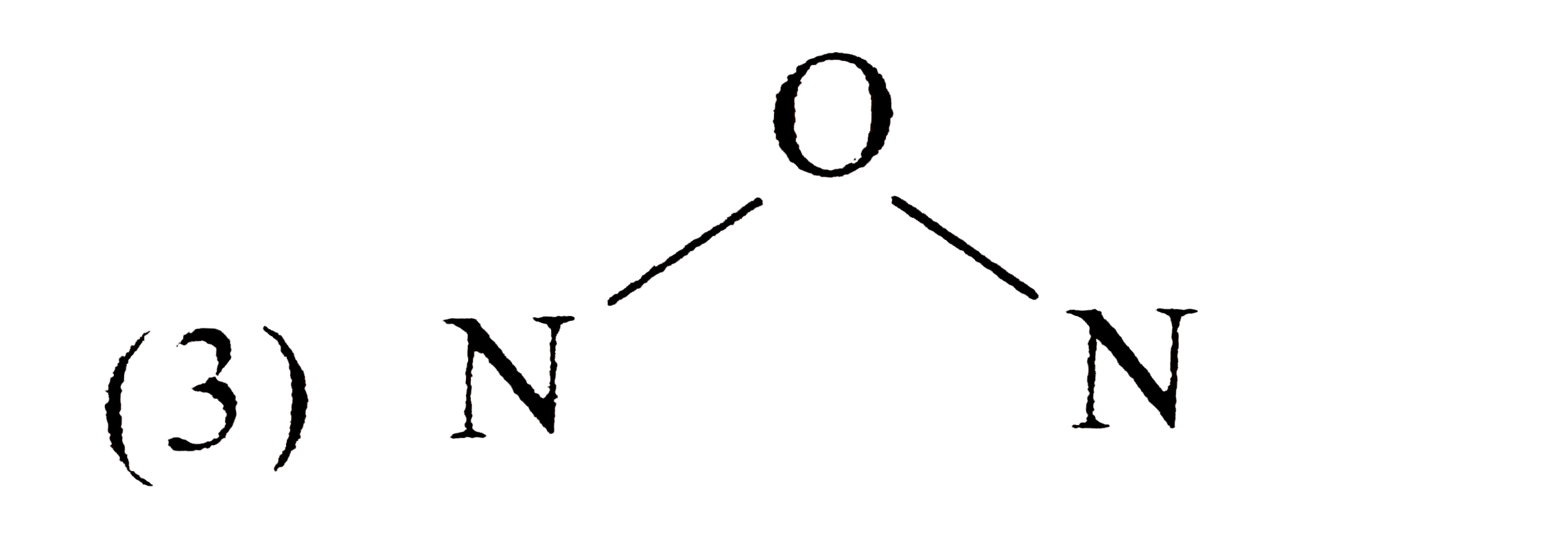
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
R SHARMA|Exercise Question Bank (Level-IV)|7 VideosCHEMICAL BONDING
R SHARMA|Exercise Archives|73 VideosCHEMICAL BONDING
R SHARMA|Exercise Question Bank (Level-II)|18 VideosAROMATIC HYDROCARBONS
R SHARMA|Exercise Archives|59 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Question Bank (Level-III)
- Which of the following oxides of nitrogen is ionic?
Text Solution
|
- Which of the following is the correct electron-dot structure of N2O mo...
Text Solution
|
- Which of the following has the highest bond dissociation enthalpy?
Text Solution
|
- The bond dissociation energy of B-F bond in BF3 is kJmol^(-1) whereas ...
Text Solution
|
- Using MO theory predict which of the following sepcies has the shortes...
Text Solution
|
- RbO2 is
Text Solution
|
- The bond angle and dipole moment of water respectively are :
Text Solution
|
- The number of nodal planes present in a sigma^** antibonding orbital i...
Text Solution
|
- Which one of the following constitutes a group of the isoelectronic sp...
Text Solution
|
- Which of the following is not paramangnetic?
Text Solution
|
- Which of the following has transient existence?
Text Solution
|
- Which of the following is the structure of N2O which is isoelectronic ...
Text Solution
|
- Which one of the following molecules is expected to exhibit diamagneti...
Text Solution
|
- The percentage of p-character in the orbitals forming p-p bonds in P4 ...
Text Solution
|
- The species having bond order different from that in CO is
Text Solution
|
- Among the following , the paramagnetic compound is :
Text Solution
|
- In which of the following ionixation processes , the bond order has in...
Text Solution
|
- How many types of F-S-F bonds are present in SF4?
Text Solution
|
- Which among the following has smallest bond angle ?
Text Solution
|