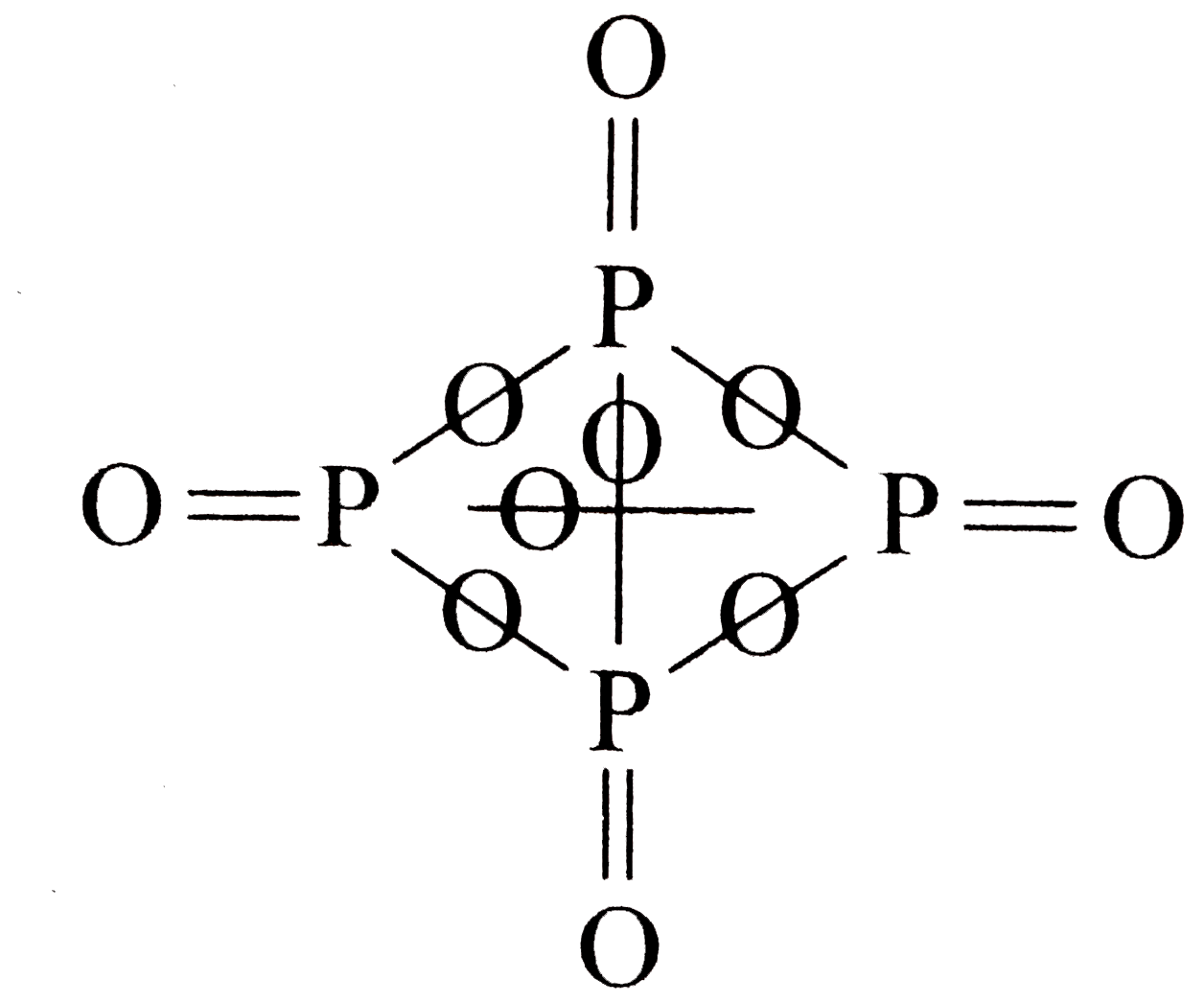
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Question Bank (Level-IV)
- The molecule of sulphuric acid contains
Text Solution
|
- The number of water molecule(s) derectly bonded to the metal centre in...
Text Solution
|
- The correct order of stabilities of the following resonance structures...
Text Solution
|
- How many sigma and pi bonds are present in the linear chain compound w...
Text Solution
|
- Stability of the species Li(2), Li(2)^(-), Li(2)^(+) increases in the ...
Text Solution
|
- A square planar complex is formed by hybridisation of which atomic ori...
Text Solution
|
- Number of sigma bonds in P4 O(10) is :
Text Solution
|