A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Archives
- The hybridization of oxygen atom in H2O2 is
Text Solution
|
- Which one of the following pairs consists of only paramagnetic species
Text Solution
|
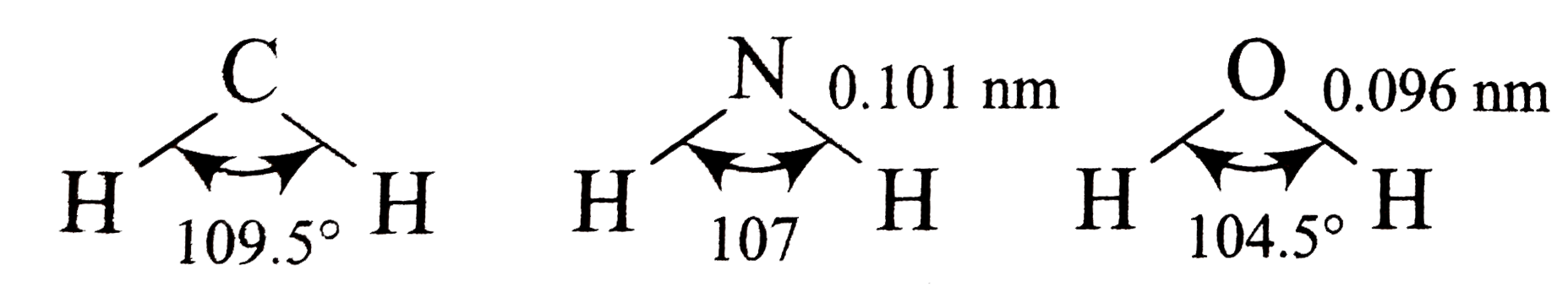
- The bond lengths and bond angles in the molecules of methane, ammonia,...
Text Solution
|
- The correct order of bond order values among the following (i) NO^(...
Text Solution
|
- A coordinate bond is a dative bond. Which of the following is true?
Text Solution
|
- In TeCl4, the central tellurium involves the hybridization
Text Solution
|
- In which of the following pairs are the two species isostructural?
Text Solution
|
- The number of sigma and pi- bonds in allyl isocyanide are
Text Solution
|
- The energy of hydrogen bond is of the order of
Text Solution
|
- Which of the following has the least bond angle?
Text Solution
|
- Match the list I and II and choose the correct matching: {:(List I(S...
Text Solution
|
- The decreasing order of the boiling points of the following hydrides ...
Text Solution
|
- Which of the following molecule is planar?
Text Solution
|
- In [Ag(CN)2]^(-), the number of pi bonds is
Text Solution
|
- Which of the following is not a correct statement?
Text Solution
|
- The number of unpaired electrons in a parmamagnetic diatomic molecule ...
Text Solution
|
- Which of the following is not isostructural with SiCI(4) ?
Text Solution
|
- Which of the following species has a linear shape ?
Text Solution
|
- In which of the following molecules all the bonds are not equal ?
Text Solution
|
- The electronegaivity difference between N and F is greater than that b...
Text Solution
|