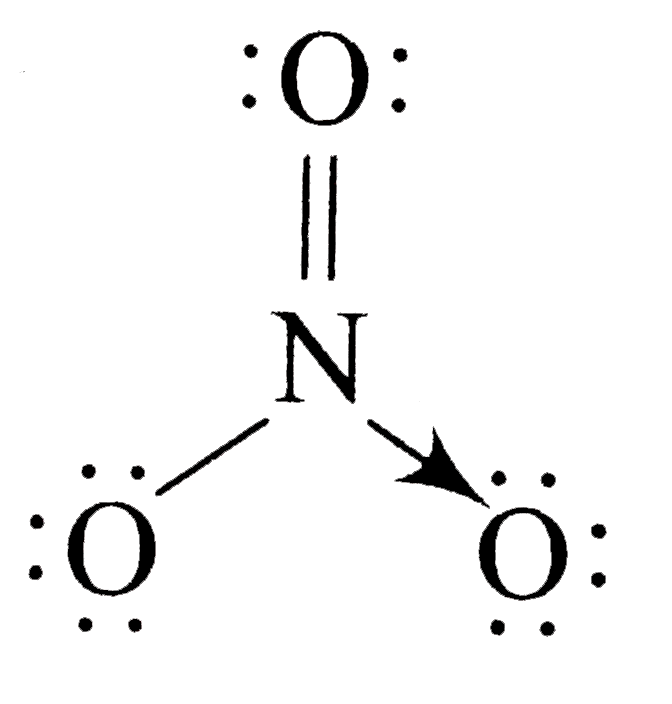
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Archives
- Shape of O2F2 is similar to that of
Text Solution
|
- Which of the following is a correct set with respect to molecule, hybr...
Text Solution
|
- Which of the following is diamagnetic?
Text Solution
|
- H2S is more acidic than H2O. The reason is
Text Solution
|
- Maximum bond angle is present in case of
Text Solution
|
- Which of the following statement is not correct for sigma and pi- bond...
Text Solution
|
- Number of pi electrons present in naphthalene is
Text Solution
|
- The electronegativities of F, Cl, Br, and I are 4.0, 3.0, 2.8, and 2.5...
Text Solution
|
- Dipole moment is shown by
Text Solution
|
- Which of the following does not contain coordinate bond?
Text Solution
|
- In OF2, the number of bond pairs and lone pairs of electrons are respe...
Text Solution
|
- In NO3^- ion, the number of bond pair and lone pair of electrons no N-...
Text Solution
|
- Which of the following has ppi-dpi bonding?
Text Solution
|
- Which of the following is soluble in water
Text Solution
|
- Which pair among the following is isostructural?
Text Solution
|
- The main axis of diatomic molecule is z. The orbitals px and py overla...
Text Solution
|
- Sideways overlap of p-p orbitals forms
Text Solution
|
- The shape of ClO3^(-) is
Text Solution
|
- The correct order of bond angles in the molecules, H2O, NH3, CH4, and ...
Text Solution
|
- Fluorine molecule is formed by
Text Solution
|