A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THERMODYNAMICS-Ouestion Bank Level IV
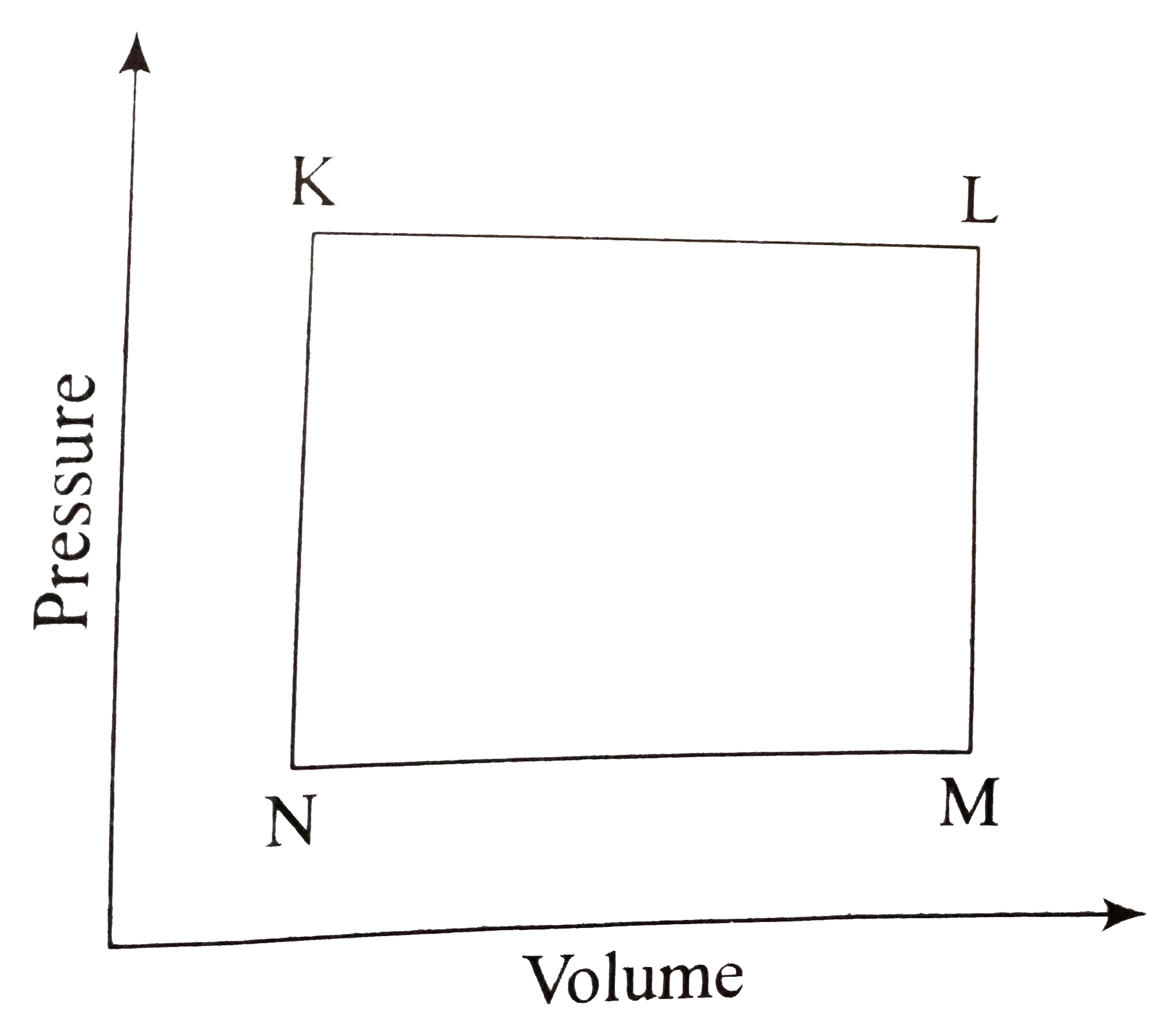
- A fixed mass m of a gas is subjected to transformation of state: K to ...
Text Solution
|
- The value of log(10)K for a reaction A hArr B is (Given: Delta(f)H(298...
Text Solution
|
- Which of the following does not express the criterion of spontanetiy?
Text Solution
|
- A porcess is nonspontaneous at evey temperature if (i) Delta H gt 0,...
Text Solution
|
- When 0.1 mol of a gas absorbs 41.75 J of heat at constant volume, the ...
Text Solution
|
- One mole of a non-ideal gas undergoes a change of state (2.0atm,3.0L,9...
Text Solution
|