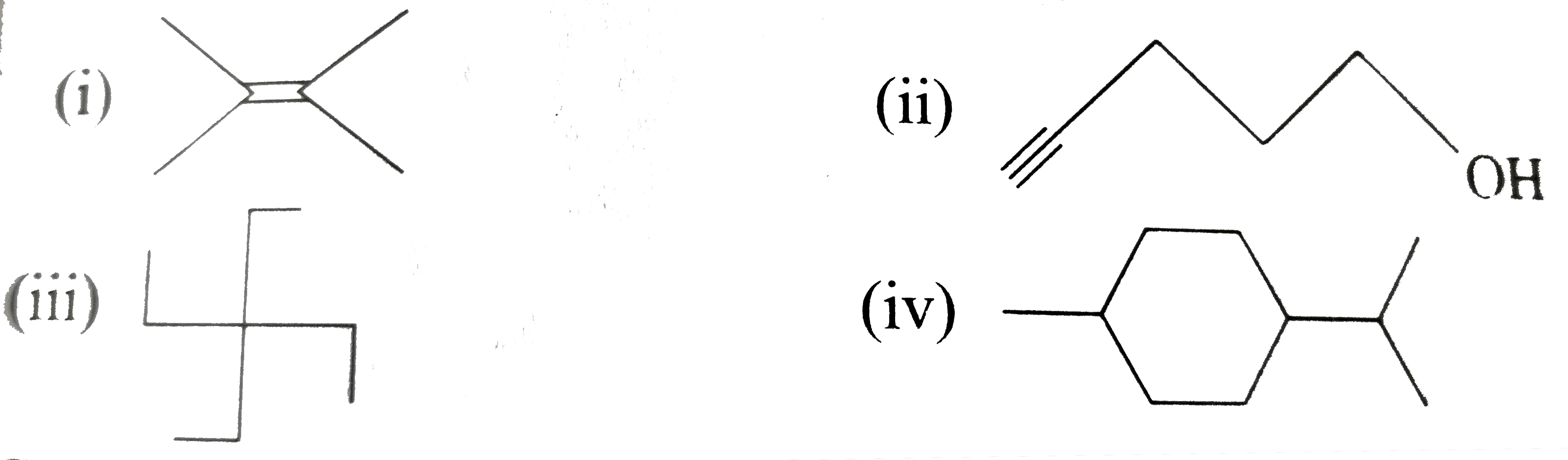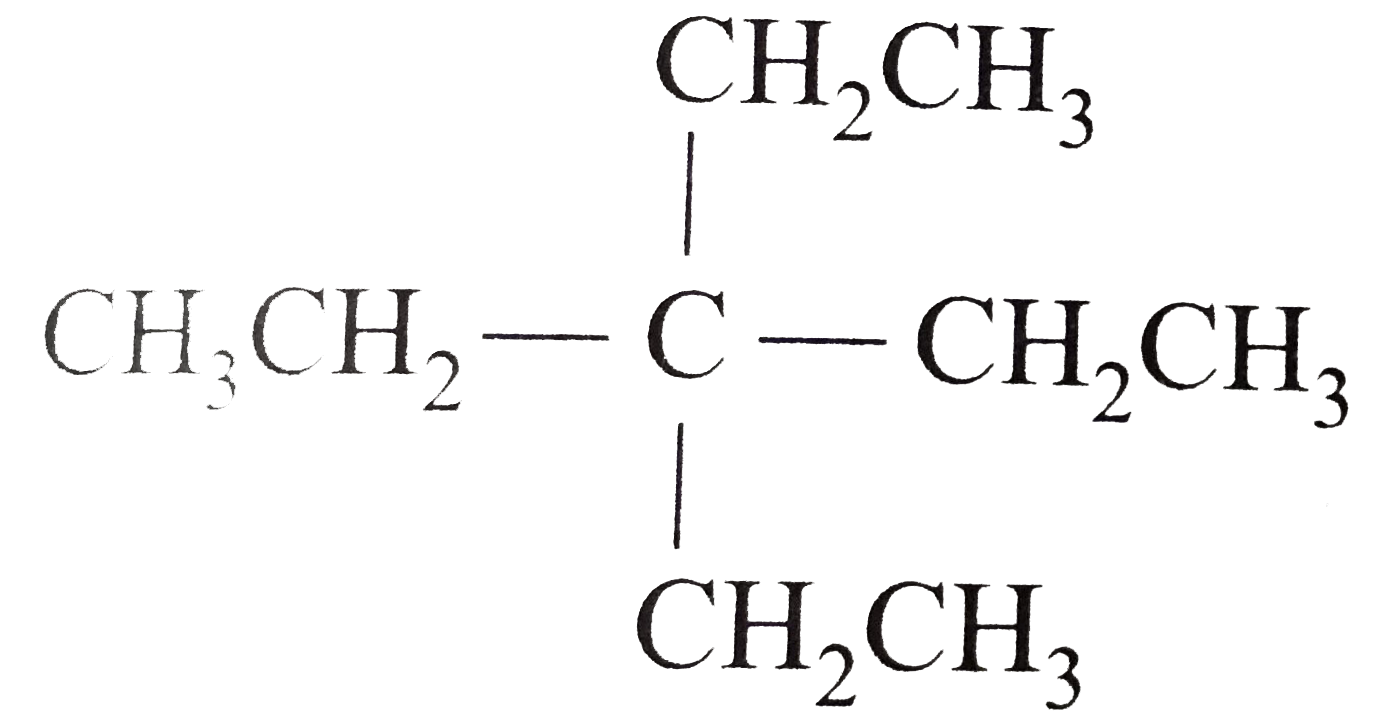Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-NOMENCLATURE OF ORGANIC COMPOUNDS-Archives
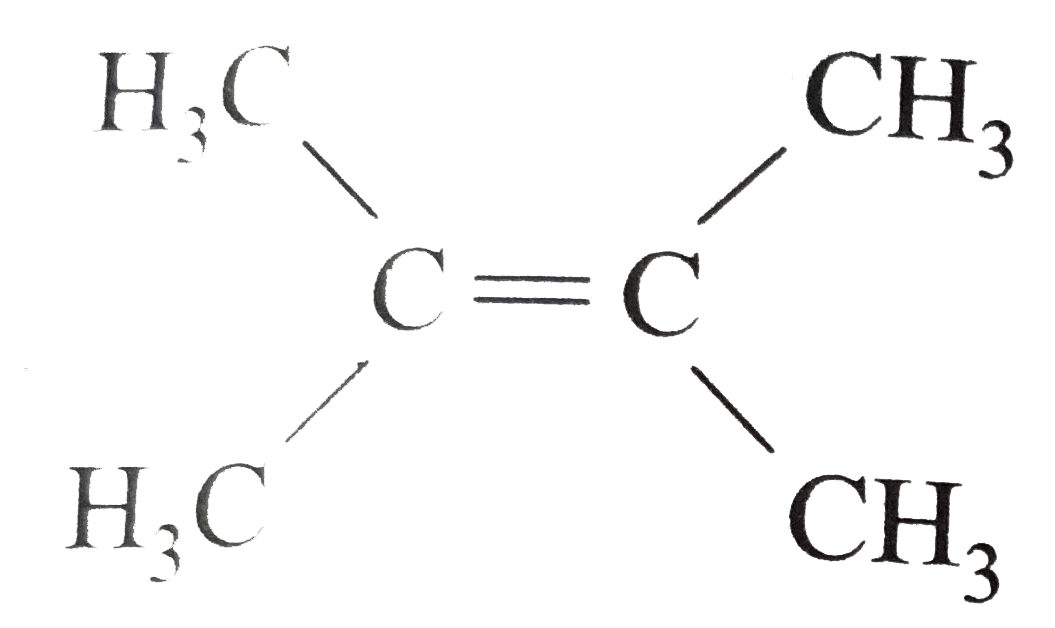
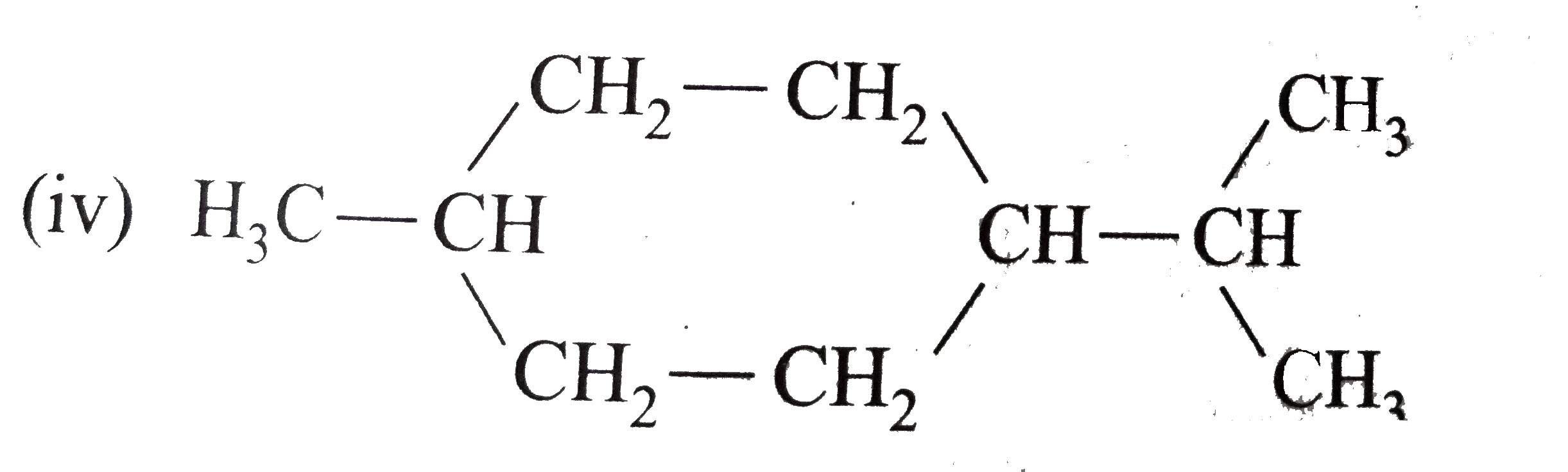
- Expand each of the following bond-line formulas to show all the possib...
Text Solution
|
- Structure of the compund whose IUPAC name is 3-ethyl-2-hydroxy-4-methy...
Text Solution
|
- The structure of isobutyl group in an organic compound is
Text Solution
|
- The IUPAC name of the compound having the formula CH-=C-CH=CH(2) is
Text Solution
|
- Consider the following compound: The IUPAC name of the this compo...
Text Solution
|
- The general molecular formula, which represents the homologous series ...
Text Solution
|
- The IUPAC name of is
Text Solution
|
- The names of some compounds are given. Which one not in the IUPAC syst...
Text Solution
|
- The IUPAC name of CH(3)CH(2)C(Br)=CHCl is
Text Solution
|
- The IUPAC name of is
Text Solution
|
- Name of the compound given below is
Text Solution
|
- The name of Cl-CH(2)-underset(Br)underset(|)(C)= underset(Br)underset(...
Text Solution
|
- The IUPAC name of 4-isopropyl-m-xylene is
Text Solution
|
- The IUPAC name of CH(3)C(CH(3))(2)CH(2)CH=CH(2) is
Text Solution
|
- The correct IUPAC name is
Text Solution
|
- The IUPAC name of CH(3)Ch(2)-underset(CH(3))underset(|)overset(H)ov...
Text Solution
|
- The IUPAC name of acraldehyde is
Text Solution
|
- The IUPAC name of CH(2)overset(OH)overset(|)(CH)CH(2)overset(CH(3))o...
Text Solution
|
- The IUPAC name of tert-butyl chloride is
Text Solution
|
- The IUPAC name of CH(3)OC(2)H(5) is
Text Solution
|
- The IUPAC name of CH(3)-overset(O)overset(||)(C)-CH(2)-overset(OH)ov...
Text Solution
|