A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NOMENCLATURE OF ORGANIC COMPOUNDS
R SHARMA|Exercise follow up test 3|16 VideosNOMENCLATURE OF ORGANIC COMPOUNDS
R SHARMA|Exercise follow up test 4|37 VideosNOMENCLATURE OF ORGANIC COMPOUNDS
R SHARMA|Exercise Follow up test 1|9 VideosISOMERISM
R SHARMA|Exercise Archives|11 VideosPURIFICATION AND CHARATERIZATION OF ORGANIC COMPOUNDS
R SHARMA|Exercise Archives|38 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-NOMENCLATURE OF ORGANIC COMPOUNDS-Follow up test 2
- Which of the following is incorrect regarding the alkanes?
Text Solution
|
- Which of the following is incorrect regarding the alkanes?
Text Solution
|
- Which of the following alkanes can exhibit cis-trans isomerism? (i) ...
Text Solution
|
- Which of the following is incorrect regarding the alkanes?
Text Solution
|
- Which of the following molecules contains the shortest C-H bonds?
Text Solution
|
- Which of the following molecules has the maximum number of pi bonds?
Text Solution
|
- Which of the following compounds contains one sp hybridized C atom?
Text Solution
|
- Which of the following is incorrect about the structural formulas?
Text Solution
|
- Which of the following is the correct dash formula for n-propyl alocoh...
Text Solution
|
- Which of the following is not correct for condensed structure formulas...
Text Solution
|
- The condensed forlmula for isoprophyl alcohol can be written in…………dif...
Text Solution
|
- Which of the following is incorrect regarding bond-line formulas ?
Text Solution
|
- Which of the following is not correct regarding the wedge-and-dashed r...
Text Solution
|
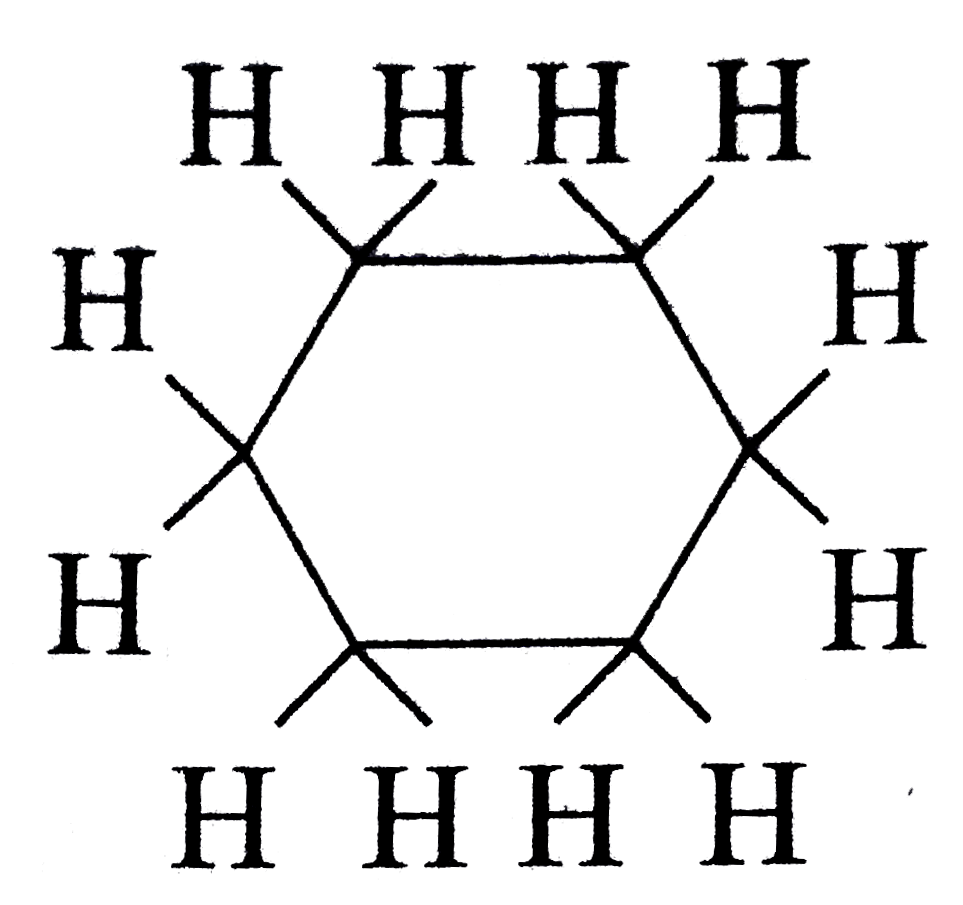 with `0 pi` bonds and
with `0 pi` bonds and 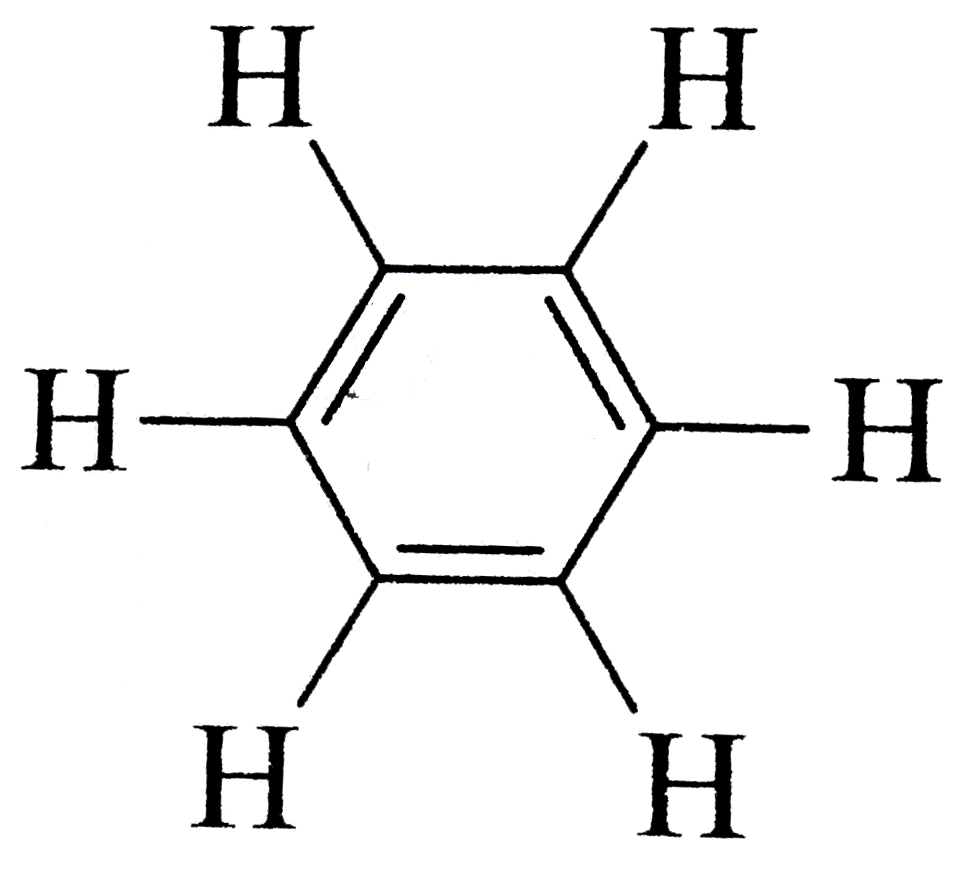 with `3 pi` bonds
with `3 pi` bonds  has `2 pi` bonds and `6 sigma`
has `2 pi` bonds and `6 sigma` 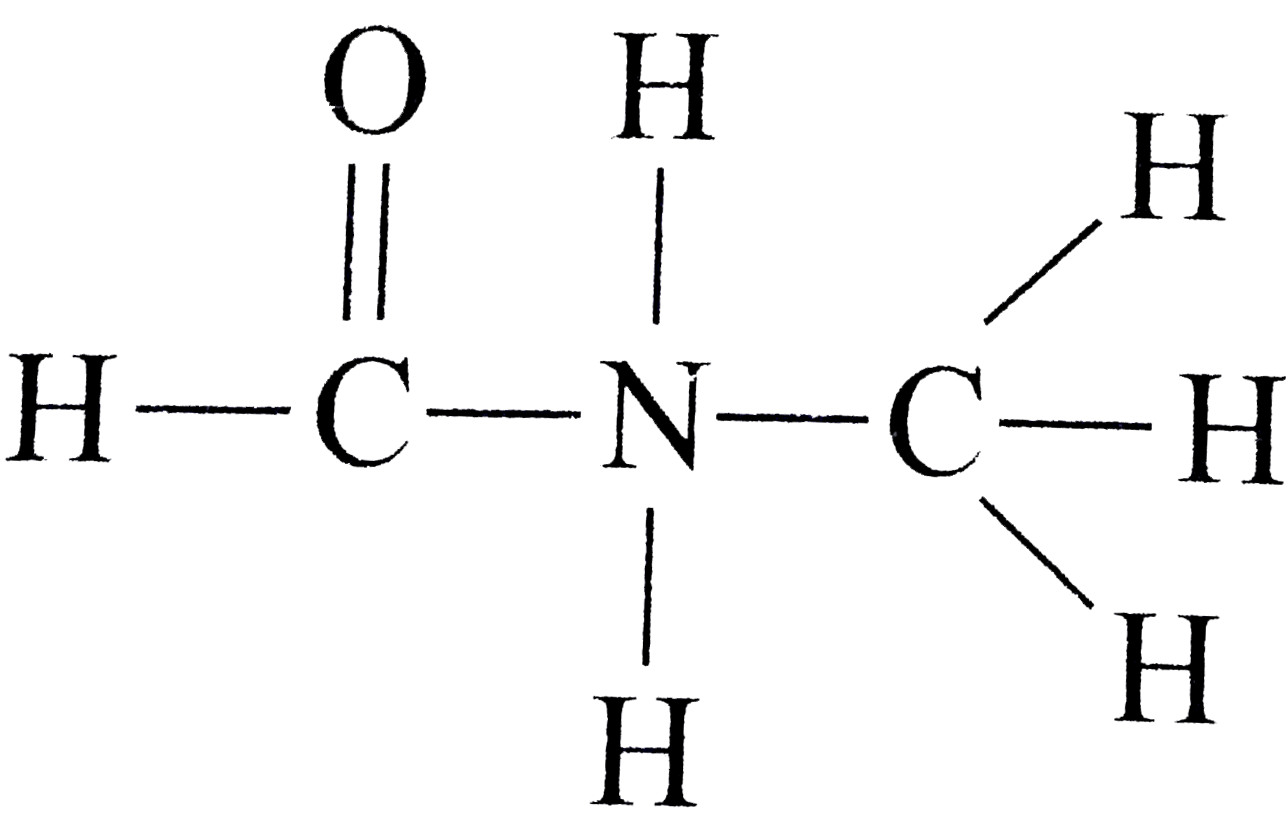 has `1 pi` bond and `9 sigma` bonds.
has `1 pi` bond and `9 sigma` bonds.