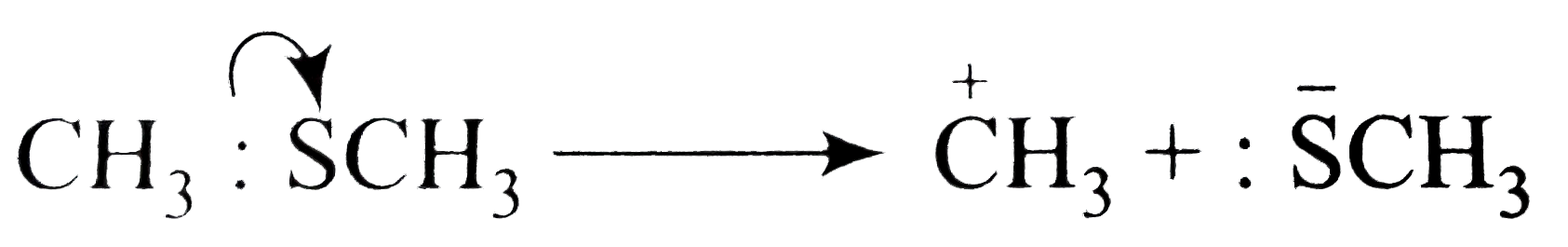
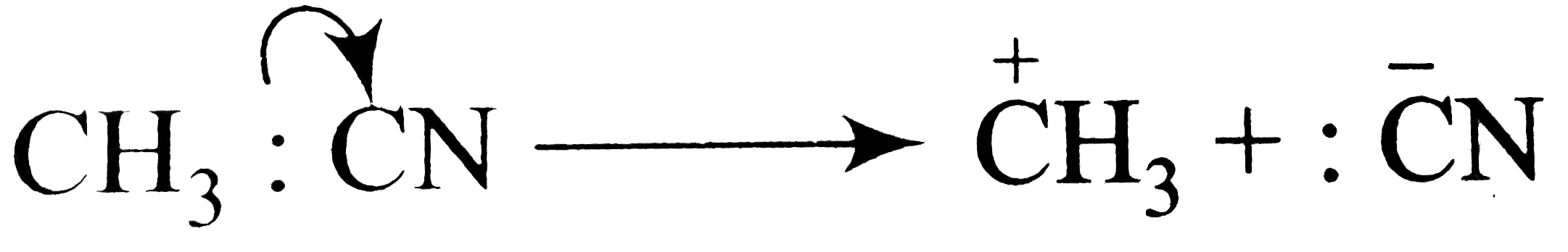
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-GENERAL ORGANIC CHEMISTRY-Archives
- Using curved-arrow notation, show the formation of reactive intermedia...
Text Solution
|
- Archives
Text Solution
|
- Arrange the following free radicals in the order of decreasing stabili...
Text Solution
|
- Base strength of the following (i) H(3)C CH(2)^(-) , (ii) H(2)C=CH^(...
Text Solution
|
- The stability of carbanions in the following (i) RC-=overset(-)overs...
Text Solution
|
- CH(3)CH(2)Cl undergoes homolytic fission to produce
Text Solution
|
- Hyperconjugation involves overlap of the following orbitals :
Text Solution
|
- Chlorination of methane in the presence of ultraviolet light involves:
Text Solution
|
- Which species are more resonance stabilized in the following pairs: ...
Text Solution
|
- The most stable carbocation is:
Text Solution
|
- Consider the following carbocation: (i) Cl(3)overset(+)C (ii) Cl(2...
Text Solution
|
- Which of the following compounds prossesses the C-H bonds with the low...
Text Solution
|
- Which of the following alkoxides is the most reactive nucleophile?
Text Solution
|
- :overset(-)CH(2)-underset( :O: )overset(C)(||)-CH(3) and CH(2)=underse...
Text Solution
|
- Resonance in most of the organic molecules
Text Solution
|
- Which of the following is the most stable?
Text Solution
|
- Polarization of electrons in acrolein may be written as:
Text Solution
|
- Nucleophilicity order is correctly represented by
Text Solution
|
- Among the following alkenes: {:(1-"Butene,",cis-2-"Butene",,"trans"-...
Text Solution
|
- Which of the following is an electrophile ?
Text Solution
|
- Which of the following is the active species in the nitration of aroma...
Text Solution
|