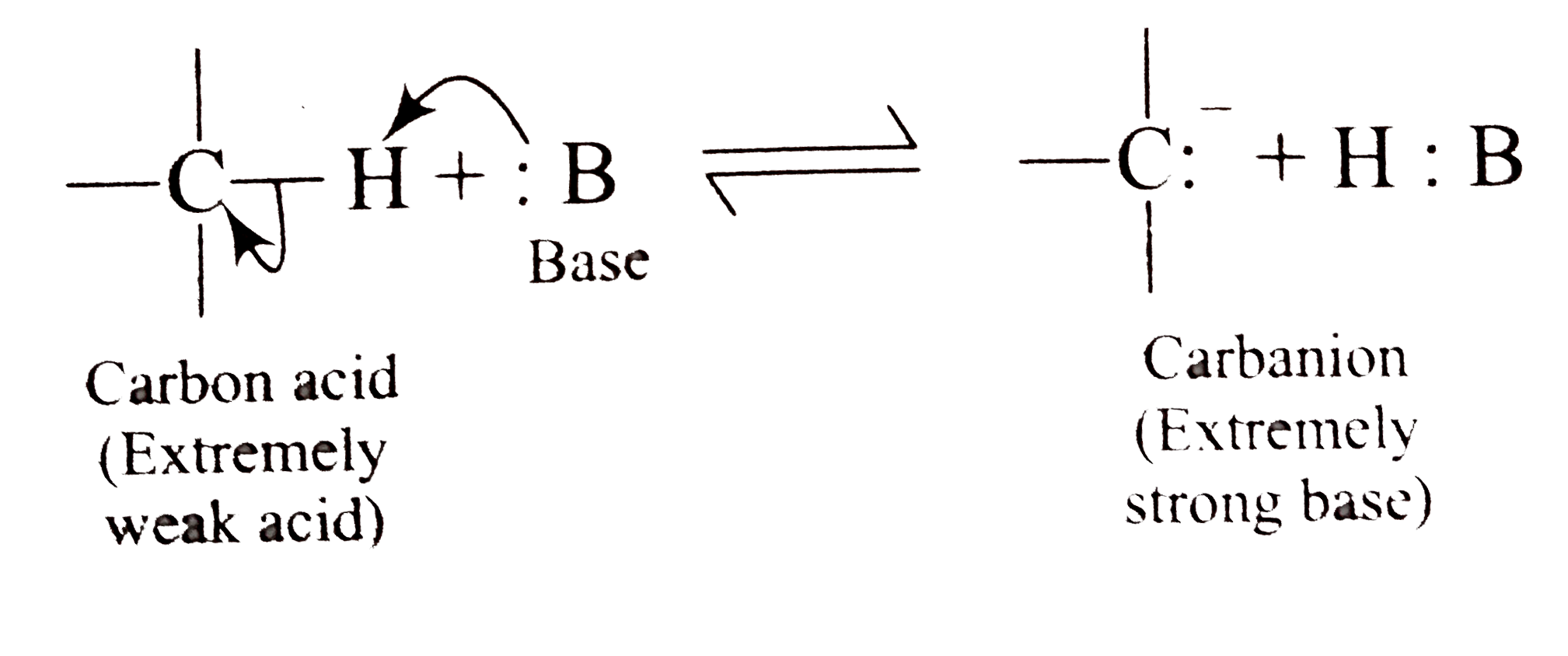A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-GENERAL ORGANIC CHEMISTRY-Follow-up test 3
- The total number of electrons present in the valance shell of the nega...
Text Solution
|
- The hybridization of the negetively charged C atom of overset(-)overse...
Text Solution
|
- The geometry of the methanide ion, overset(-)overset(..)CH(3),
Text Solution
|
- The H-C-H bond angle in overset(-)overset(..)CH(3), is
Text Solution
|
- Which of the following can serve as a source from which C atom is read...
Text Solution
|
- Which of the following is correct for a carbon?
Text Solution
|
- The "" hydrogen atoms of carbonyl compounds (i.e aldehydes and ketones...
Text Solution
|
- Which of the following compounds is the most acidic
Text Solution
|
- Which of the following carbanions is the most reactive?
Text Solution
|