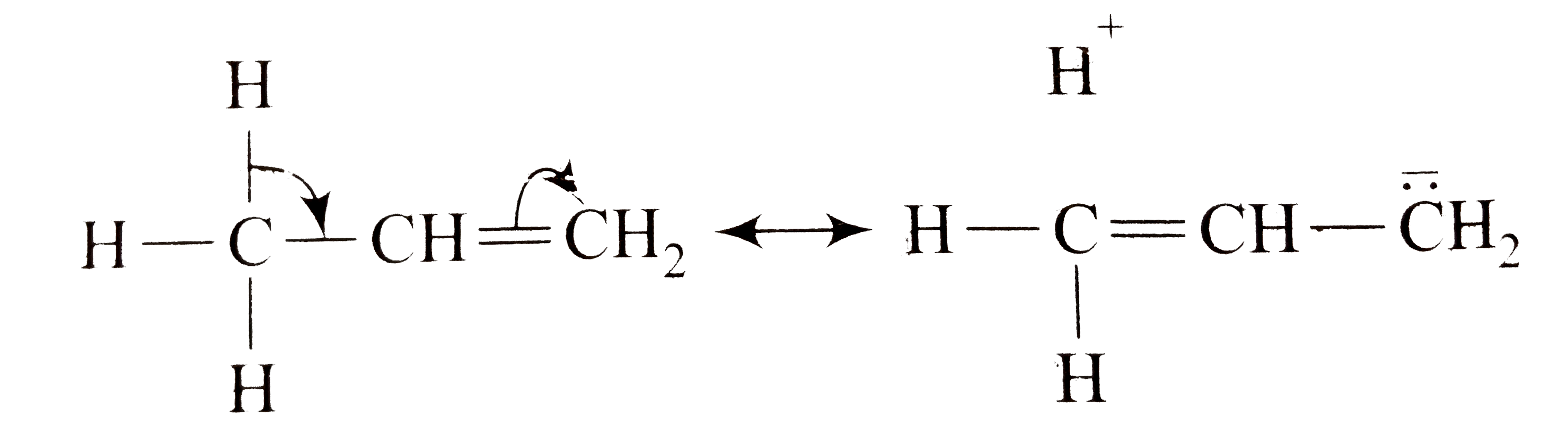A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Follow- up Test 9|6 VideosGENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Question bank Level - I|1 VideosGENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Follow- up Test 7|11 VideosEQUILIBRIUM
R SHARMA|Exercise ARCHIVES|69 VideosISOMERISM
R SHARMA|Exercise Archives|11 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-GENERAL ORGANIC CHEMISTRY-Follow- up Test 8
- Which of the following does not exhibit electromeric effect?
Text Solution
|
- Which of the following electrons displacement effects is temporary?
Text Solution
|
- Which of the following electronic effects does not take place in the g...
Text Solution
|
- The kind of delocalisation involving sigma bond orbitals is called………....
Text Solution
|
- The CH(3)- group in propene exerts
Text Solution
|
- The total number of contributing structures for hyperconjugation in pr...
Text Solution
|
- Which of the following electron displacement effects in covalent bonds...
Text Solution
|
- Which of the following alkyl groups exerts maximum hyperconjugation wh...
Text Solution
|
- Which of the following cannot exhibit hyperconjugation?
Text Solution
|