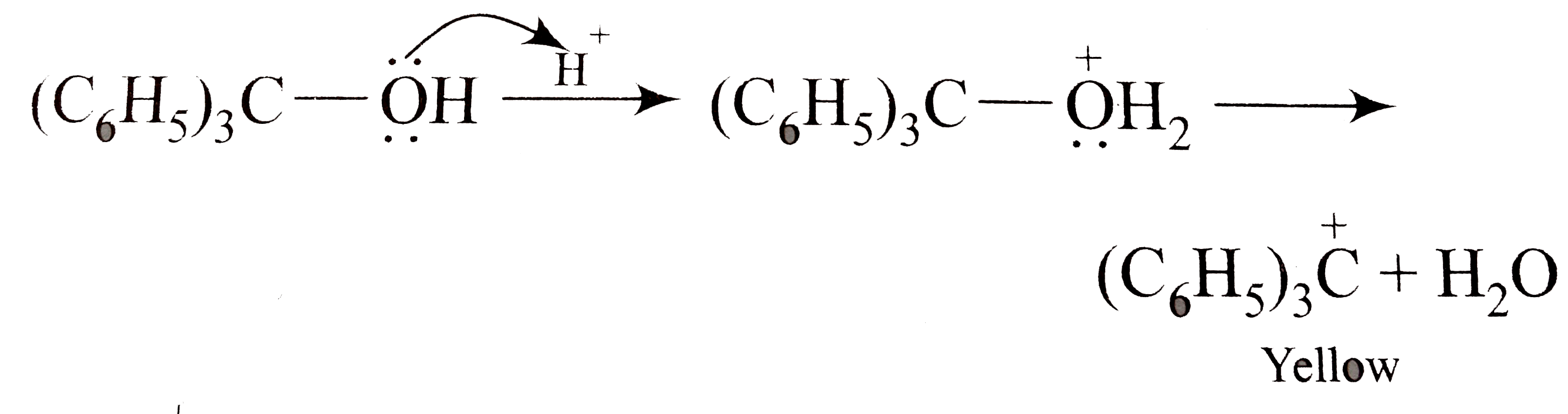A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-GENERAL ORGANIC CHEMISTRY-Level - III
- Among the following, the least stable resonance structure is :
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following would yied a carbanion to the greatest extent o...
Text Solution
|
- The treatment of Me(3)C-F with SbF(5) in liquid SO(2) would yield a
Text Solution
|
- In which of the following molecule/ions are the inductive effect, reso...
Text Solution
|
- In the following carbocation, H//CH3 that is most likely to migrate to...
Text Solution
|