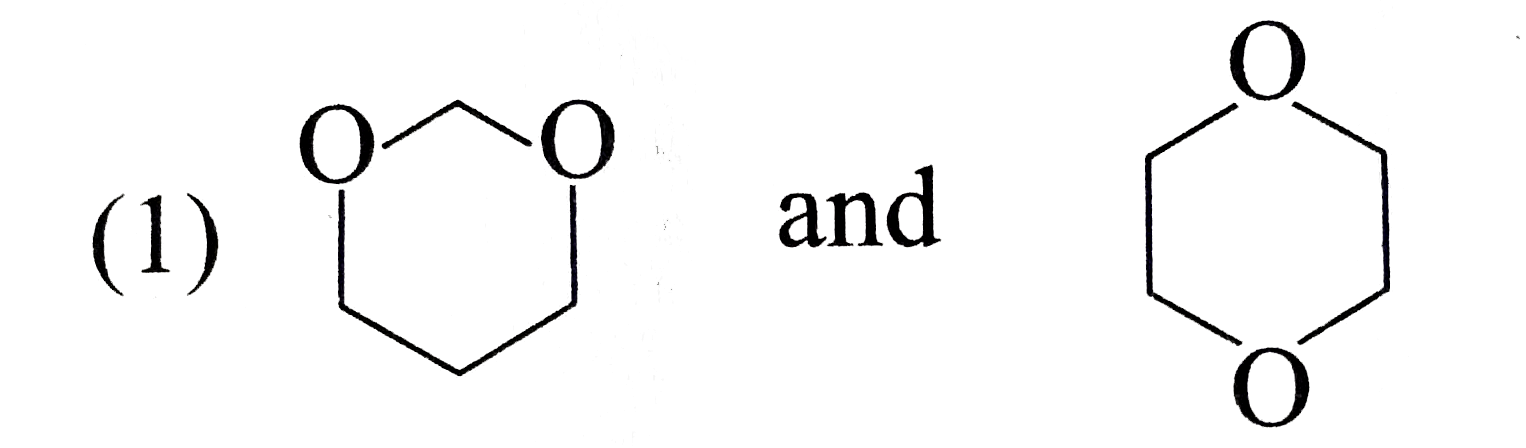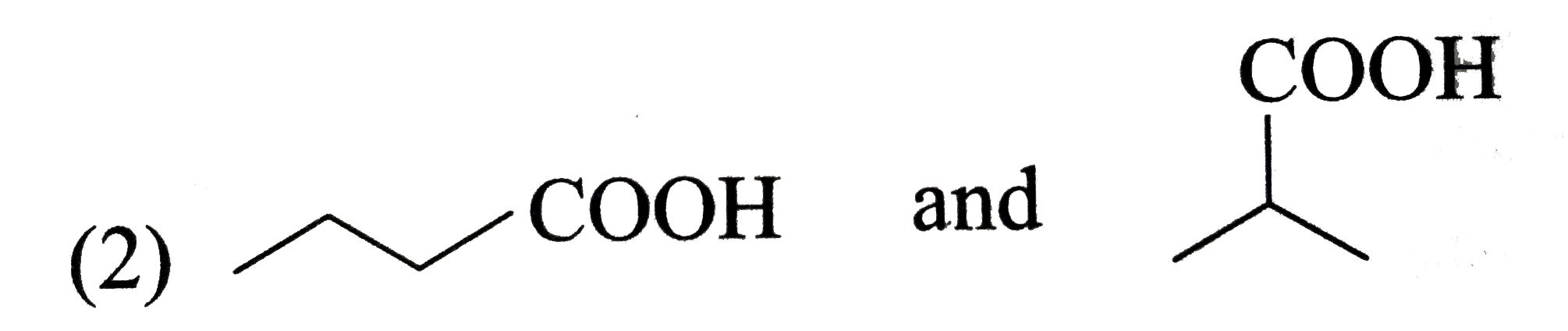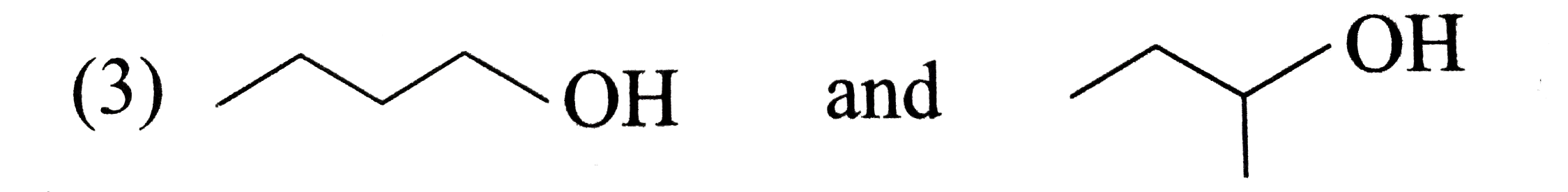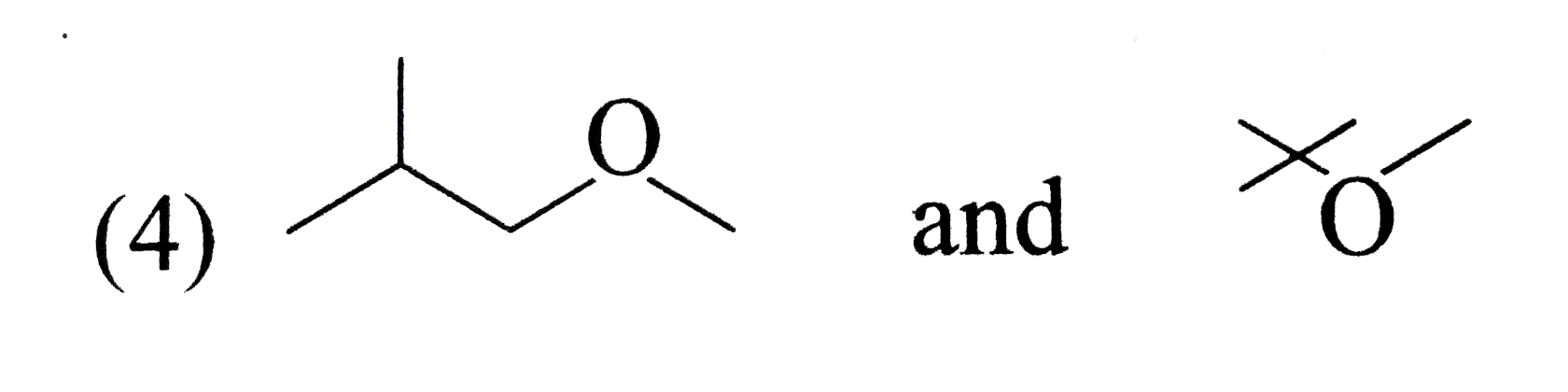A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ISOMERISM-Follow-up Test 2
- How many constututional isomers are possible for the alkene C4H8?
Text Solution
|
- Which of the statements are correct for alkyne with molecular formula ...
Text Solution
|
- Which of the statements are correct for alkyne with molecular formula ...
Text Solution
|
- Which of the statements are correct for alkyne with molecular formula ...
Text Solution
|
- How many dichloro derivatives (only constitutional isomers) of propane...
Text Solution
|
- How may trichloro derivatives (only constitutional isomers) of propane...
Text Solution
|
- How many choropentanes (C5H(11)Br2) are possible if only structural is...
Text Solution
|
- How many dibromobutanes (C4H8Br2) are possible if only structural isom...
Text Solution
|
- Which of the following alkanes gives two monochloro derivatives?
Text Solution
|
- Which of the following pairs of compounds are position isomers?
Text Solution
|
- Which of the following compounds exhibit chains as well as position is...
Text Solution
|
- Which of the following pairs of compounds are not position isomers?
Text Solution
|
- How many following pairs of compounds are not position isomers?
Text Solution
|
- Which of the following pairs of compounds are not position isomers?
Text Solution
|