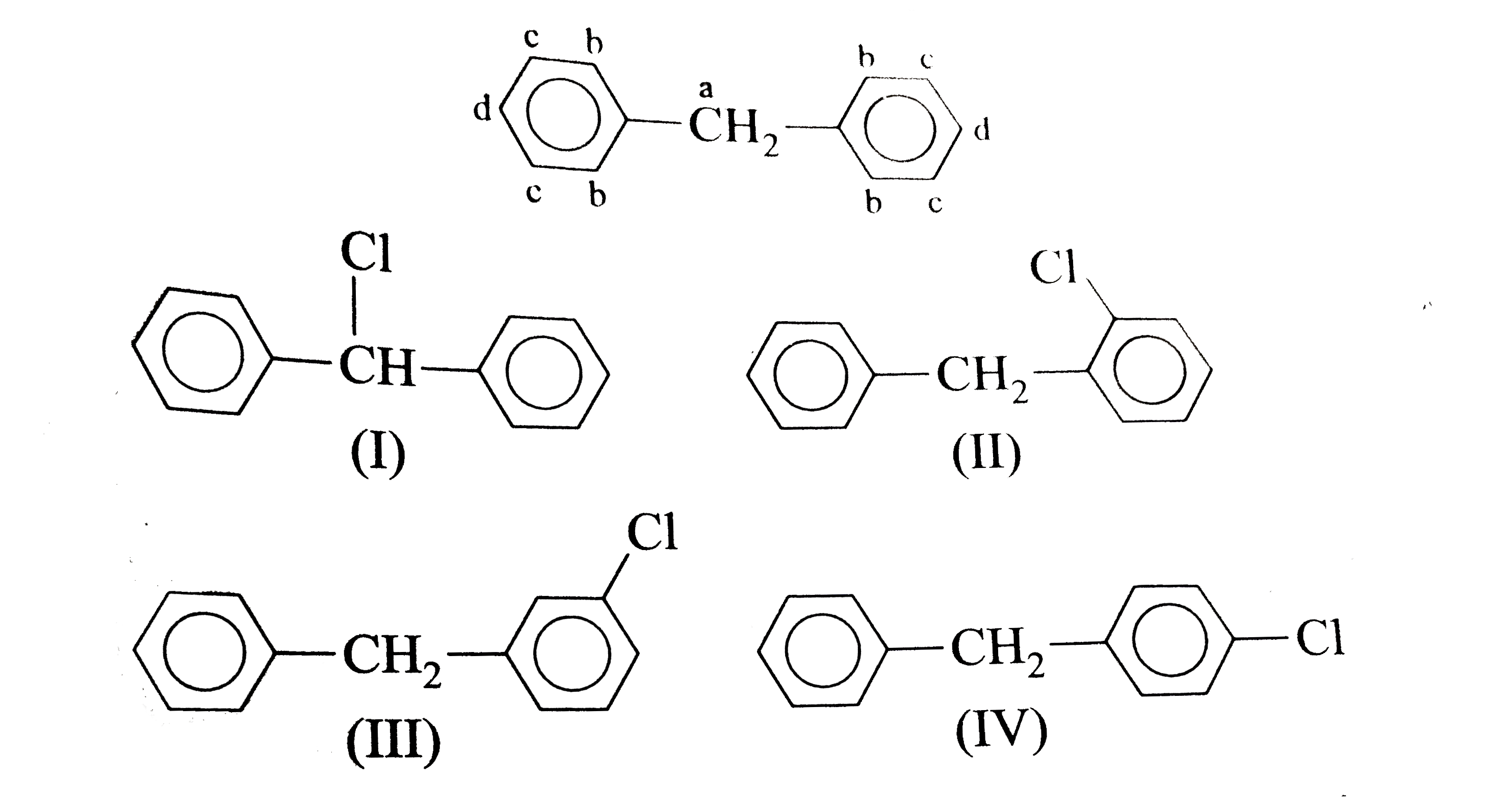
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ISOMERISM-Archives
- The order of stability of the following tautomeric compounds is (i)....
Text Solution
|
- Which isomer of hexane has only two different sets of structurally equ...
Text Solution
|
- Which one of the following compounds cannot show tautomerism?
Text Solution
|
- How many primary amines including stereoisomers are possible for the m...
Text Solution
|
- The molecular formula of diphenylmethane, How many structural isomers ...
Text Solution
|
- Isomers of propionic acid are
Text Solution
|
- The compound which is not isomeric with diethyl ether is :
Text Solution
|
- Cyanides and isocyanides are isomers of the type
Text Solution
|
- Tautomerism will be exhibited by
Text Solution
|
- n-Propyl alcohol and isopropyl alcohol are examples of
Text Solution
|
- The number of isomers of C(6)H(14) is:
Text Solution
|