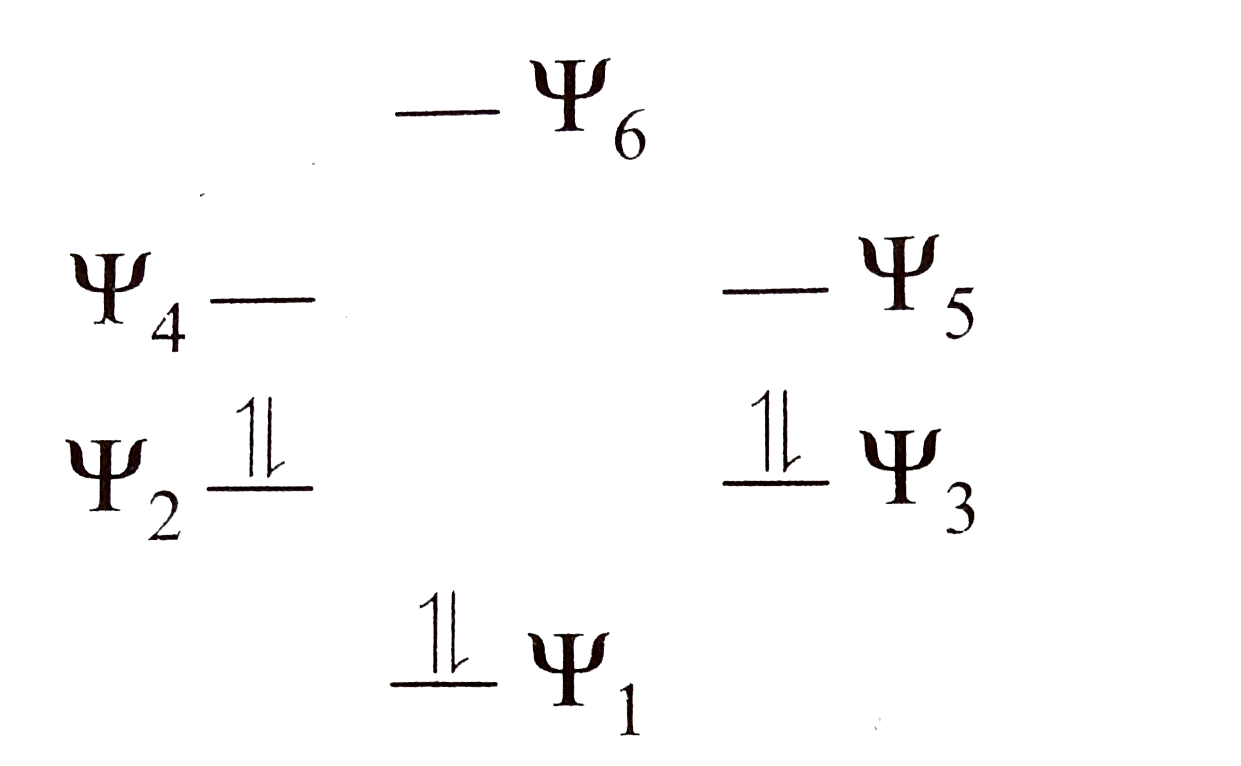A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-AROMATIC HYDROCARBONS-Follow up Test 2
- Which of the following compounds does not decolorize the red-brown col...
Text Solution
|
- The index of hydrogen deficiency (IHD) of benzene is
Text Solution
|
- The carbon-carbon bond order in benzene is
Text Solution
|
- The planar regular hexagonal structure of benzene was first proposed b...
Text Solution
|
- The number of monosubstitution products of Dewar (I), prismane (II), a...
Text Solution
|
- Consider the following structures of formula C(6)H(6) underset((I))(...
Text Solution
|
- Benzene adds on ozone to form a
Text Solution
|
- One mole of toluene on being treated with an excess of ozone and subse...
Text Solution
|
- Benzene does not respond positively to the test of unsaturation becaus...
Text Solution
|
- How resonating structures are possible for benzene?
Text Solution
|
- The C-H bond length in benzene is
Text Solution
|
- Find the heat of hydrogenation of cyclohexene if the heat of hydrogena...
Text Solution
|
- The IUPAC name of the product P is
Text Solution
|