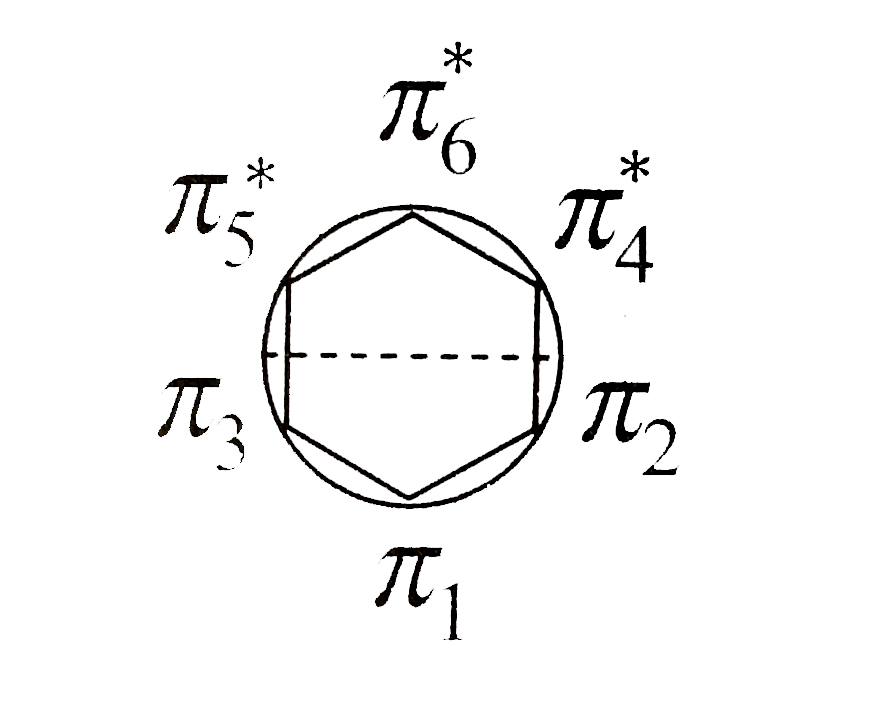A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-AROMATIC HYDROCARBONS-Follow up Test 3
- The Huckel rule for aromaticity connects aromatic stability (High delo...
Text Solution
|
- Which of the following is not Huckel number?
Text Solution
|
- The smallest aromatic species is a/an
Text Solution
|
- Which of the followig is aromatic like benzene?
Text Solution
|
- Accoding to the polygon rule, how many nonbonding nolecular orbitals a...
Text Solution
|
- How many pi electrons are present in cyclobutadienyl anion (C(4)H(4))^...
Text Solution
|
- How may Kekule-type resonance structures canbe drawn for cyclooctatetr...
Text Solution
|
- Which of the following is correct?
Text Solution
|