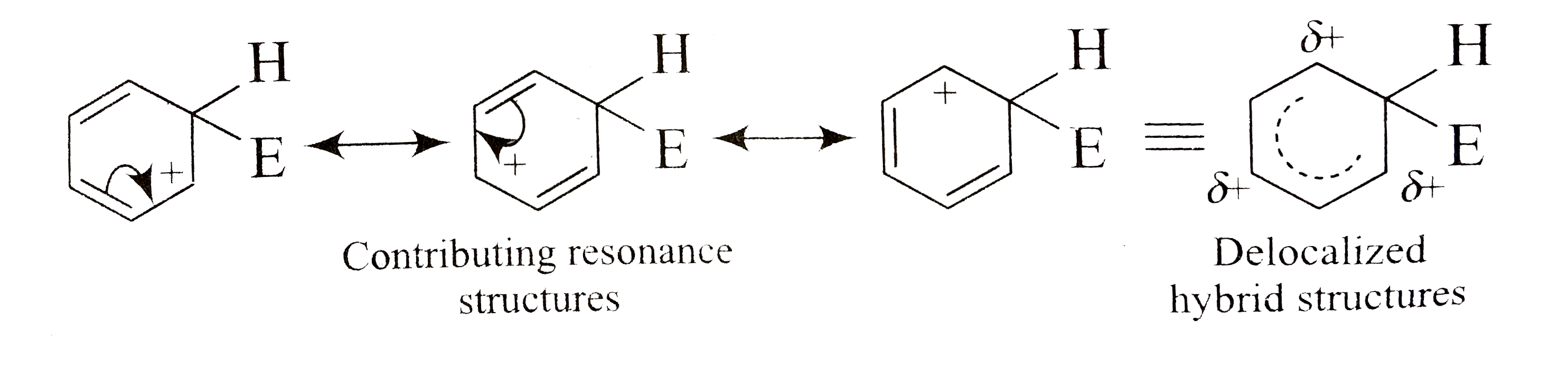A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-AROMATIC HYDROCARBONS-Follow up Test 5
- Which of the following is an arene?
Text Solution
|
- Among the three isometric trimethylbenzenes: (i). 1,2,3 (ii) 1,2,4...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- What types of reagents cause characteristic benzene substitution? (i...
Text Solution
|
- What is the first step in the reaction of an electrophile (E^()) with ...
Text Solution
|
- The benzenonium ion is a
Text Solution
|
- Which of the following is the correct enthalpy diagramfor the electrop...
Text Solution
|
- Which of the following reacts the fastest in electrophilic aromatic su...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following electrophilic aromatic sustitution is reversibl...
Text Solution
|
- Which of the following electrophilic aromatic substitution is expected...
Text Solution
|