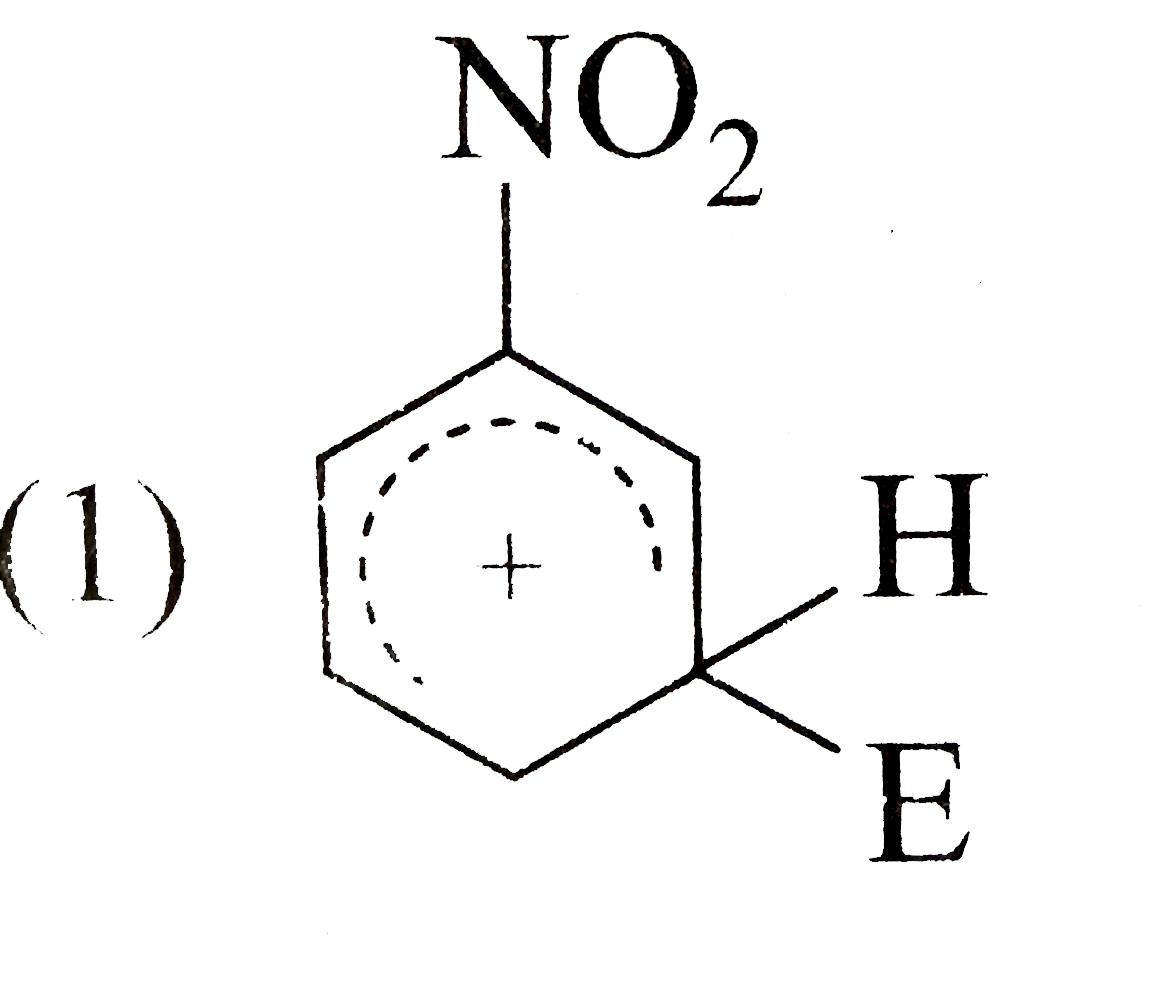
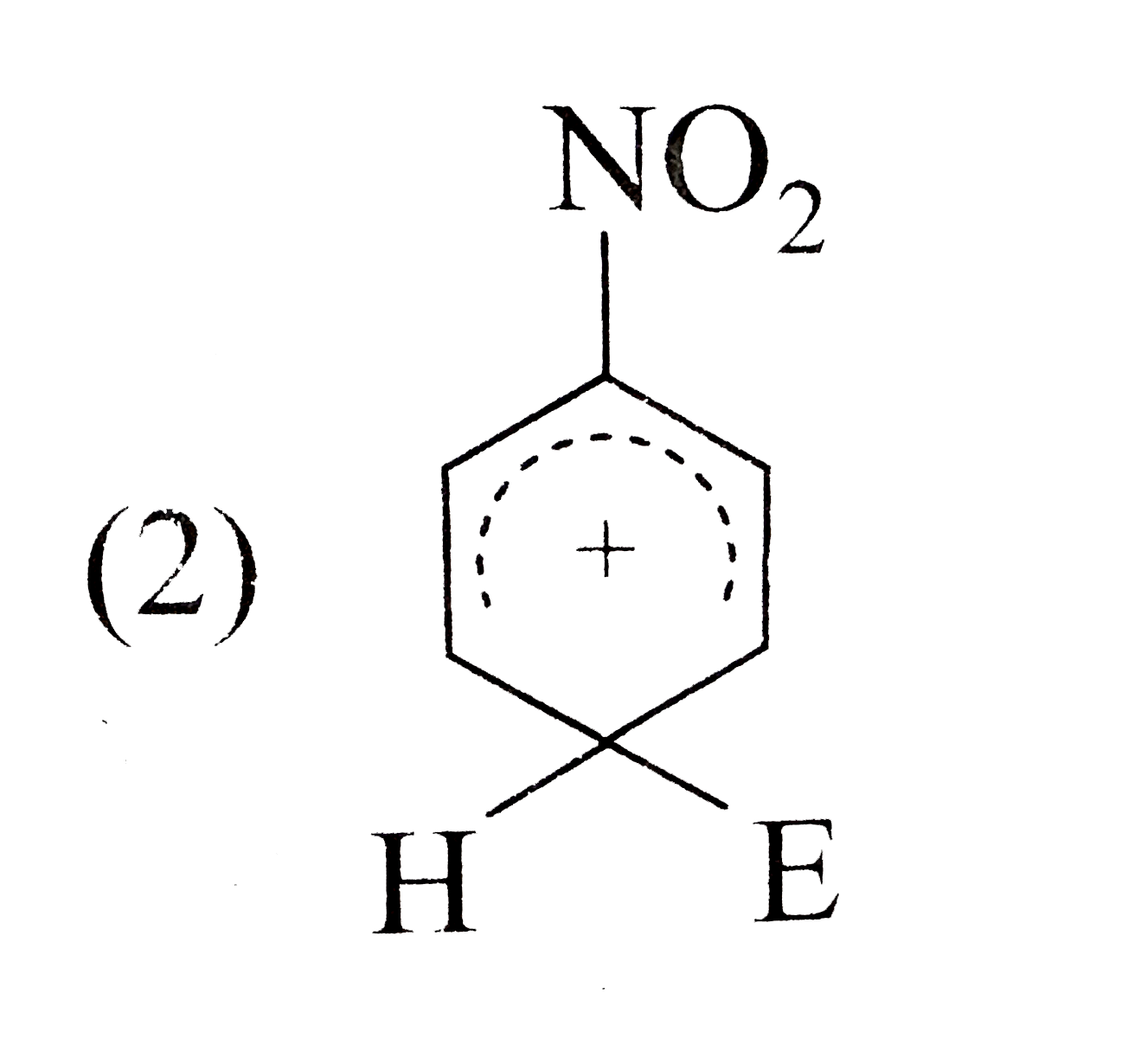
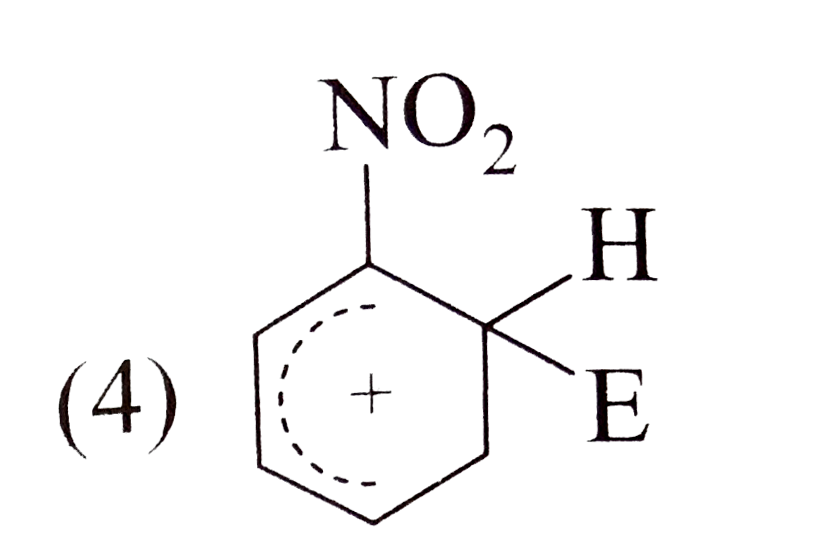
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-AROMATIC HYDROCARBONS-Follow up Test 7
- Identify the product B:
Text Solution
|
- The electrophile, E^((o+)) attacks the benzene ring to generate the in...
Text Solution
|
- Ethylbenzene with bromine in the presence of FeBr(3) predominantly giv...
Text Solution
|
- Identify the correct order of reactivity in electrophilic substitution...
Text Solution
|
- The reaction of toluene with CI(2) in presence of FeCI(3) gives predom...
Text Solution
|
- Toluene, when treated with (Br(2))/(Fe), gives p-bromotoluene as the m...
Text Solution
|
- The major product formed in the reaction is
Text Solution
|