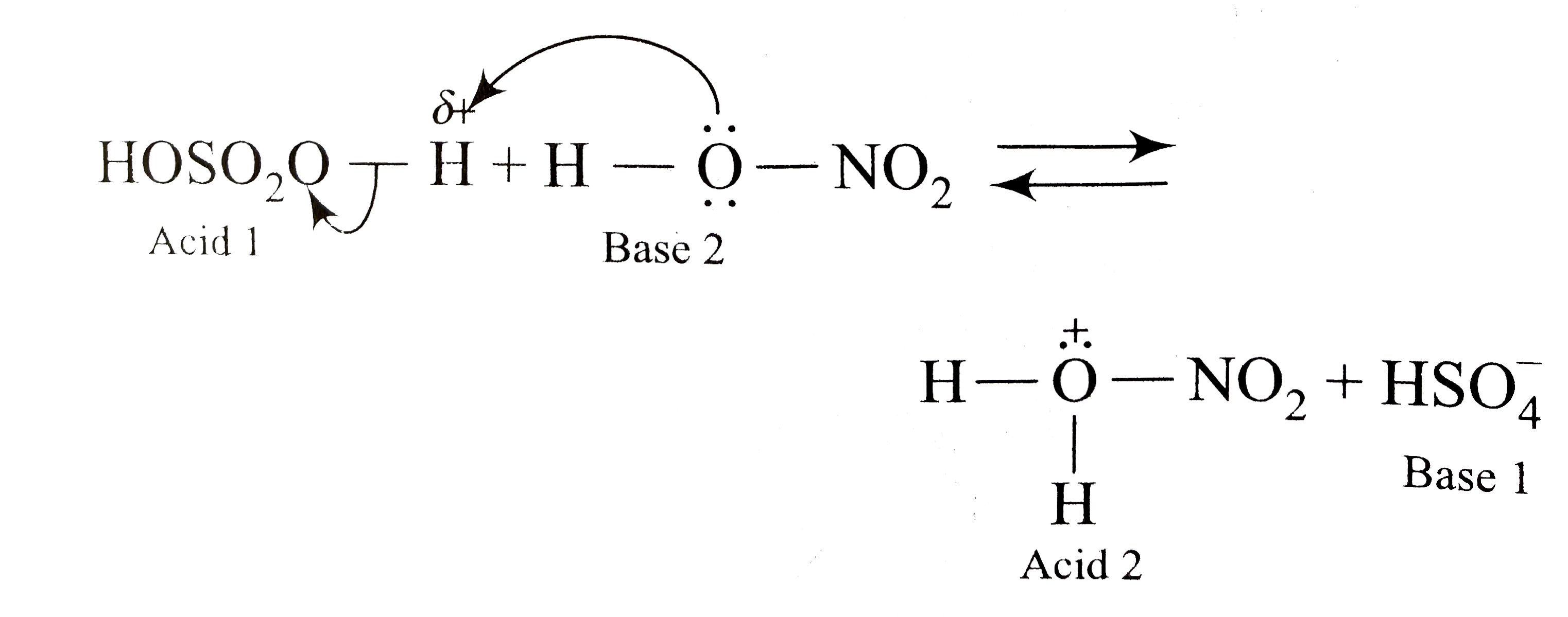A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-AROMATIC HYDROCARBONS-Archives
- In the reaction given below, X is C(6)H(5)MgBr+CH(3)OHrarrX
Text Solution
|
- Benzene reacts with CH(3)Cl in the presence of anyhydrous AlCl(3) to f...
Text Solution
|
- Nitrobenzenen can be prepared from benzene by using a mixture of conc ...
Text Solution
|
- Which of the following is not aromatic?
Text Solution
|
- In a compound electrophilic substitution has occurred the substituent...
Text Solution
|
- Which one of the followingis the most reactive towards electrophilic a...
Text Solution
|
- The order of decreasing reactivity towards an electrphilic reagent for...
Text Solution
|
- In which reaction , polysubstitution takes place :
Text Solution
|
- The major product formed on monobromination ((Br(2))/(FeBr(3))) of the...
Text Solution
|
- The strongest ortho/para and the strongest meta directing groups, resp...
Text Solution
|
- Among the following, the aromatic compound is
Text Solution
|
- The treatment of benzene with isobutene in the presence of sulphuric a...
Text Solution
|
- C-C bond length in benzene is
Text Solution
|
- The C-C-C bond angle in benzene is
Text Solution
|
- The centric formula of benzene was proposed by
Text Solution
|
- The ratio of sigma and pi bond in benzene is
Text Solution
|
- According to the huckel rule, planar and completely conjugated monocyc...
Text Solution
|
- Select the correct statement about benzene:
Text Solution
|
- Which of the following statements is not compatible with arenes?
Text Solution
|
- Aromatic character of benzene can be explained by
Text Solution
|