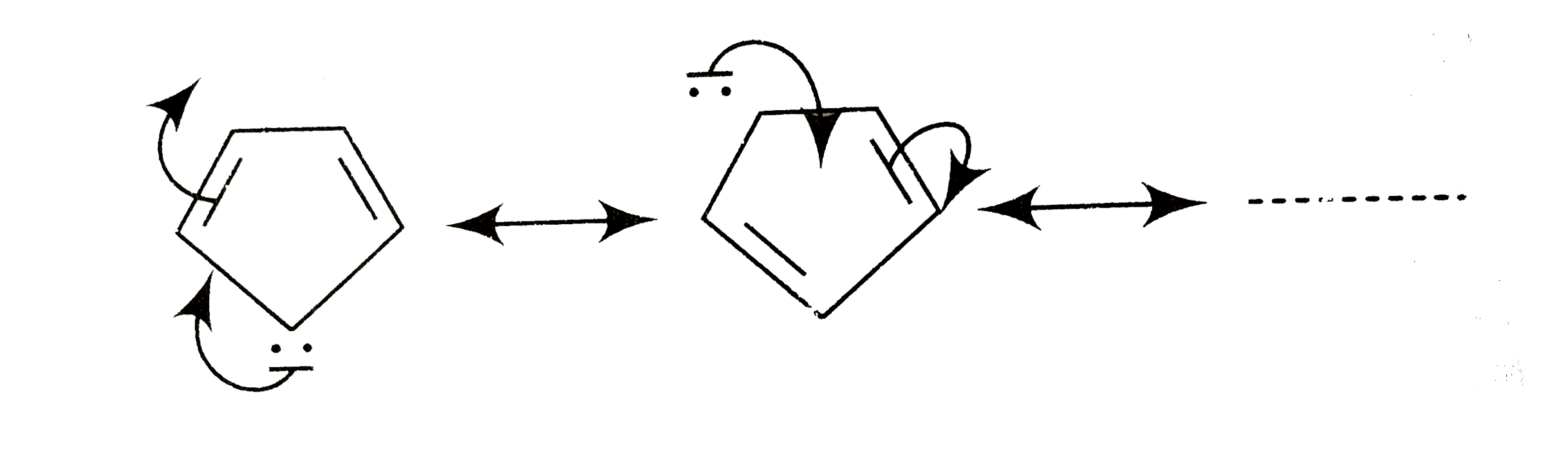
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-AROMATIC HYDROCARBONS-Archives
- Aromatic character of benzene can be explained by
Text Solution
|
- The total number of possible isomeric trimethylbenzenes is
Text Solution
|
- How many pi electrons are present in the cyclopentadienyl anion C(5)H(...
Text Solution
|
- Which of the following chemical systems is/are nonaromatic?
Text Solution
|
- Number of pi electrons present in naphthalene is
Text Solution
|
- When sodium benzoate is heated with sodalime, the product formed is
Text Solution
|
- The nitration of benzene is an example of
Text Solution
|
- In the nitration of benzene with a mixture of concentrated HNO(3) and ...
Text Solution
|
- When phenol is heated with zinc dust the major product formed is
Text Solution
|
- Which of the following compounds is the most reactive towards nitratio...
Text Solution
|
- The strongest ortho-and para-directing group among the following is
Text Solution
|
- C(6)H(6)+CI(2)overset(UVLight)rarr Product In above reaction product i...
Text Solution
|
- Among the compounds the order of decreasing reactivity towards electro...
Text Solution
|
- The function of anhydrous AlCl(3) in friedel-Crafts' reaction is to
Text Solution
|
- Benzene reacts with n-propyl chloride in the presence of anhydrous AlC...
Text Solution
|
- Which one of the following will be the most easiliy attacked by an ele...
Text Solution
|
- The most common reaction of benzene is
Text Solution
|
- In the chlorination of benzene with Cl(2), in the presence of anhydrou...
Text Solution
|
- The correct order of decresing reactivity of anisole (I), benzene (II)...
Text Solution
|
- Anhydrous AlCl(3) is used as a catalyst in the friedel-crafts reaction...
Text Solution
|