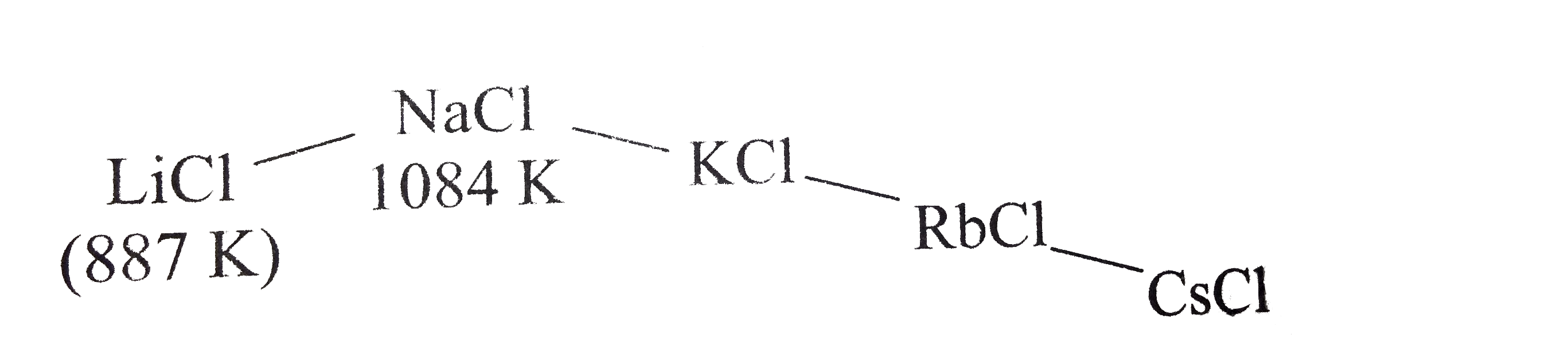
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THE S BLOCK ELEMENTS-Follow-up Test 4
- Which of the following is correct for monoxides (normal oxides) of alk...
Text Solution
|
- On combustion in excess ofd air, lithium forms
Text Solution
|
- Which of the following in incorrect for the hydroxides of alkali metal...
Text Solution
|
- All alkali metal peroxides contain the [-O-O]^(2-) ion. They are
Text Solution
|
- All alkali metal superoxides contain the ion [O(2)]. They are
Text Solution
|
- The alkali metal halides (MX) are all high melting, colorless crystall...
Text Solution
|
- Alkali metal halides have high negative enthalpies of formation. The D...
Text Solution
|
- The magnitude of enthalphy of formation of alkali metal halides decrea...
Text Solution
|
- Which of the following is soluble in organic solvents like ethanol ?
Text Solution
|
- Which of the following has the maximum melting point but minimum solub...
Text Solution
|
- Which of the following is the correct order of hyrated ionic radii ?
Text Solution
|
- Which of the following compounds is almost insoluble in water ?
Text Solution
|
- The melting and boiling points of alkali metal halides always follow t...
Text Solution
|
- Which of the following are oxosalts ? (i) Carbonates , (ii) Nitrates...
Text Solution
|
- Which of the following is nott true?
Text Solution
|
- Which of the following alkali metal carbonates doesnot existin the sol...
Text Solution
|
- Sodium bicarbonate (NaHCO(3)) on gentle heting produces
Text Solution
|
- Which of the following has the maximum solubility in water?
Text Solution
|