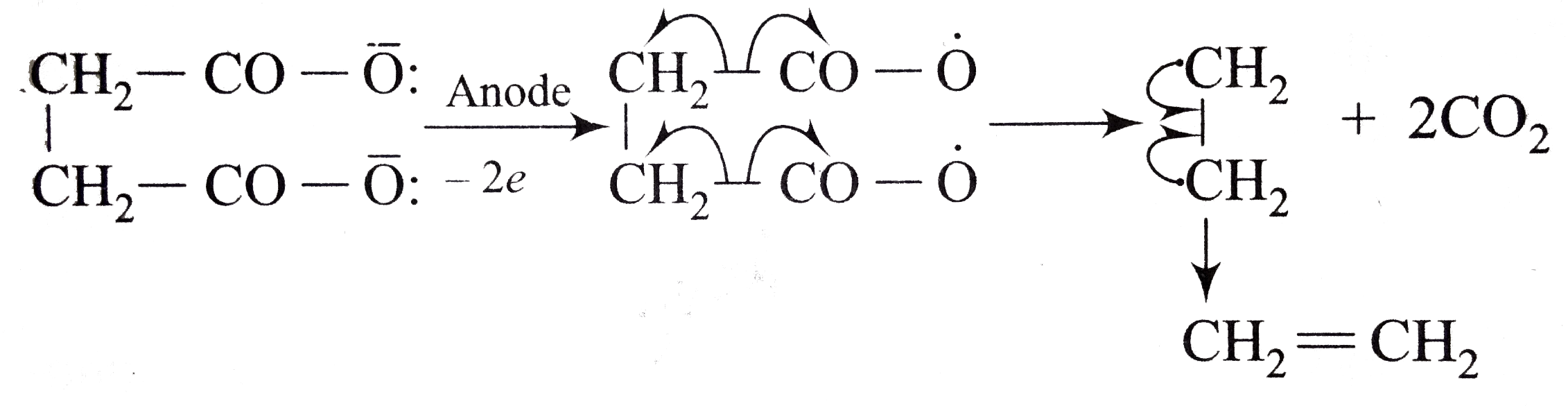
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALKENES-Follow-up-5
- The reaction BrCH(2)CH(2)Br+Znoverset(CH(3)CO(2)H)rarrCH(2)=CH(2)+Zn...
Text Solution
|
- 1,3-Diiodopropane is heated with zinc dust in ether. The product forme...
Text Solution
|
- Consider the thermal decomposition CH+(3)CH(2)underset(CH(3))underse...
Text Solution
|
- In the reaction (CH(3))(2)CH-underset(CH(3))underset(|)(CH)-overset(...
Text Solution
|
- Which of the following reagents is required in the synthesis of alkene...
Text Solution
|
- Consider the following sequence of reactions: The final product i...
Text Solution
|
- Which of the following is known as original Lindlar's catalyst?
Text Solution
|
- But-2-ene reacts with H(2) in the presence of Lindlar's catalyst. The ...
Text Solution
|
- The electrolysis of an aqueous solution of sodium." "produces ethene.
Text Solution
|