Similar Questions
Explore conceptually related problems
Recommended Questions
- A student takes 50 g wax (specific heat =0.6 kcal//kg^(@)C) and heats ...
Text Solution
|
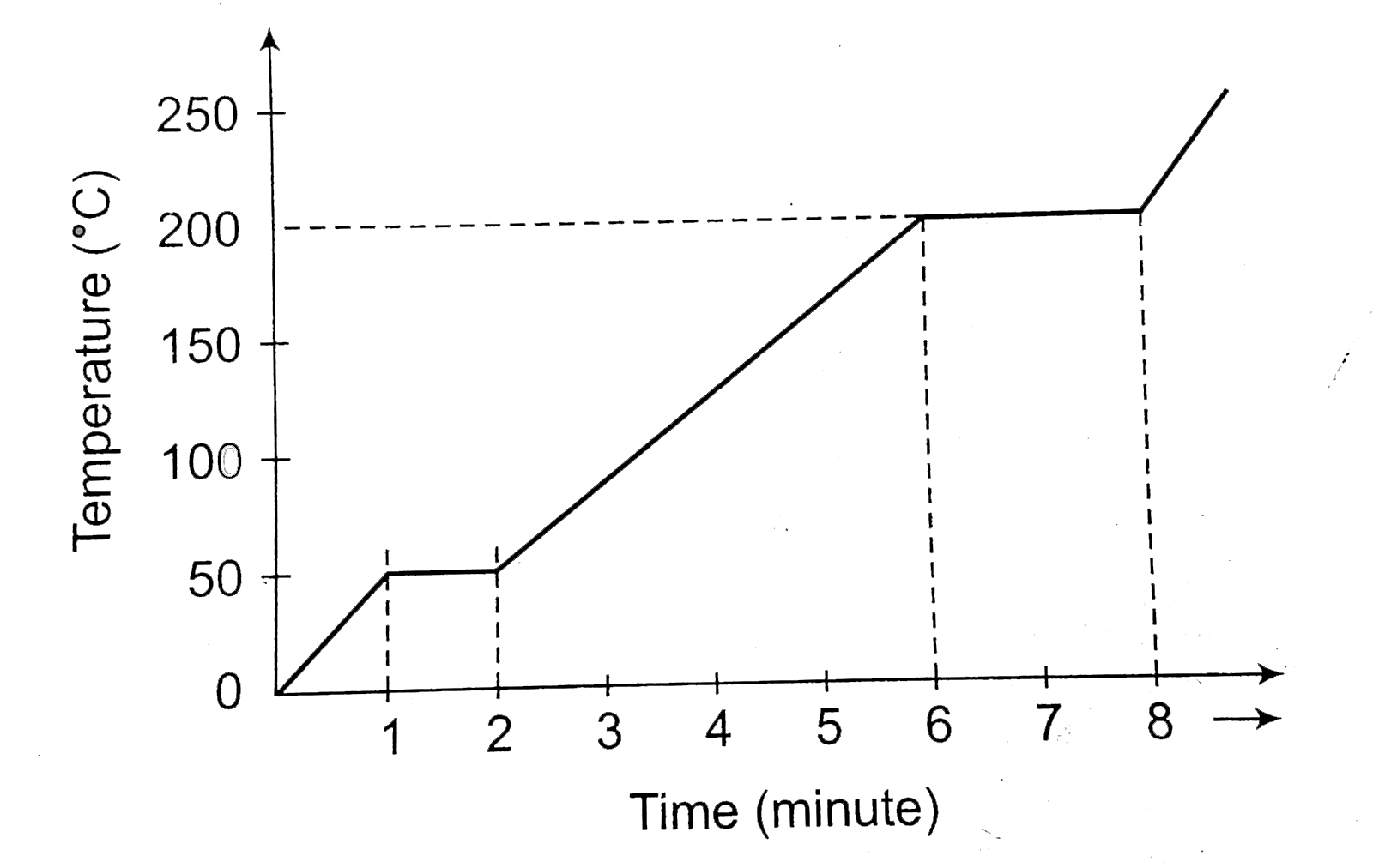
- Water at 0^@C was heated until it started to boil and then until it al...
Text Solution
|
- A student takes 50 g wax (specific heat =0.6 kcal//kg^(@)C ) and heats...
Text Solution
|
- A 20gm bullet whose specific heat is 5000 J / ( kg-.^(@)C ) and moving...
Text Solution
|
- Find the heat energy required to boil 5 kg of water iff its initial te...
Text Solution
|
- A graph is drawn by taking the rise in temperature on Y axis and heat ...
Text Solution
|
- The specific heat of water at boiling point is
Text Solution
|
- A calorimeter has mass 80 g and specific heat 0.1 kcal/kg 0 C. It cont...
Text Solution
|
- A calorimeter has mass 80 g and specific heat 0.1 kcal/kg 0 C. It cont...
Text Solution
|