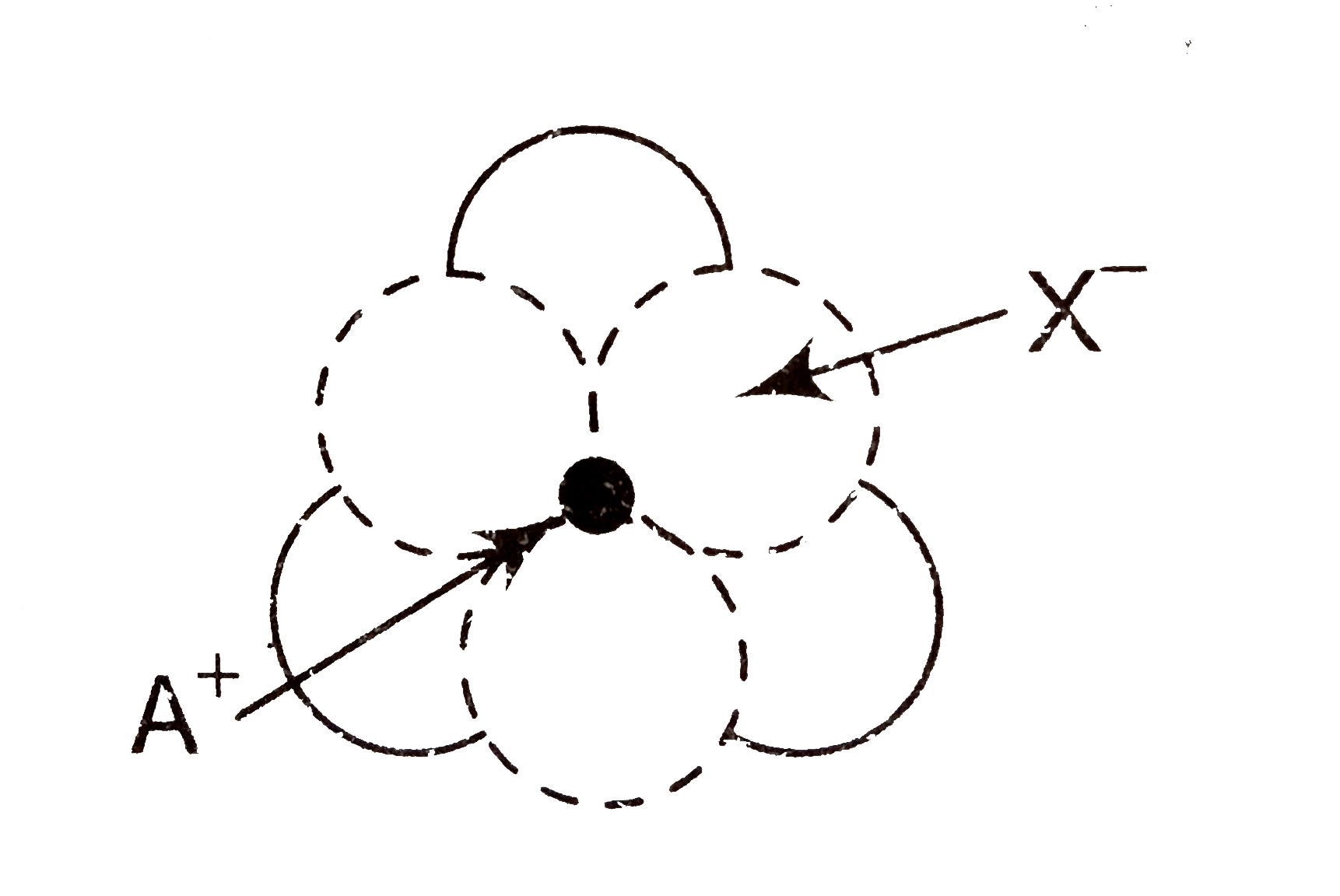A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-SOLID STATE-QUESTION BANK level 2
- If Z is the number of atoms in the unit cell that represent the closed...
Text Solution
|
- A metal crystallises in a bcc lattice ,its unit cell edge length in ab...
Text Solution
|
- In face -centered cubic unit cell, edge length is
Text Solution
|
- On doping Ge with a little of In or Ga one gets
Text Solution
|
- A compound is formed by elements A and B. This crystallises in the cub...
Text Solution
|
- A semiconductor of Ge can be made p-type by adding
Text Solution
|
- Which of the crystal systems has maximum number of Bravais lacttices?
Text Solution
|
- Ruby is an example of crystal with aa chemical impurity .The crystal i...
Text Solution
|
- Experimentally it was found that a metal oxide in formula M(0.98)O. Me...
Text Solution
|
- The second order Bragg diffraction of X rays with lambda=Å form a set ...
Text Solution
|
- A pure crystallic substance , on being heated gradually first a hurbit...
Text Solution
|
- AgBr can exhibit
Text Solution
|
- The Ca^(2+) and F^(-) ions arc located in CaF(2) crystal respectively ...
Text Solution
|
- if a=b ne c and alpha=beta=gamma=90^(@), the crystal system is
Text Solution
|
- The crystal system of a compound with unit cell dimensions a=0.387,b=0...
Text Solution
|
- In a compound XY(2) O(4) , oxide ions are arranged in CCP and cations ...
Text Solution
|
- Which of the following is used ot make magnetic tapes for use in audio...
Text Solution
|
- CsCl crystallizes in body centred cubic lattice .If 'a' is its edge le...
Text Solution
|
- The arrangement of X^(-) ions around A^(+) in solids AX is given in th...
Text Solution
|
- The liquefied metal expanding on soildification is
Text Solution
|