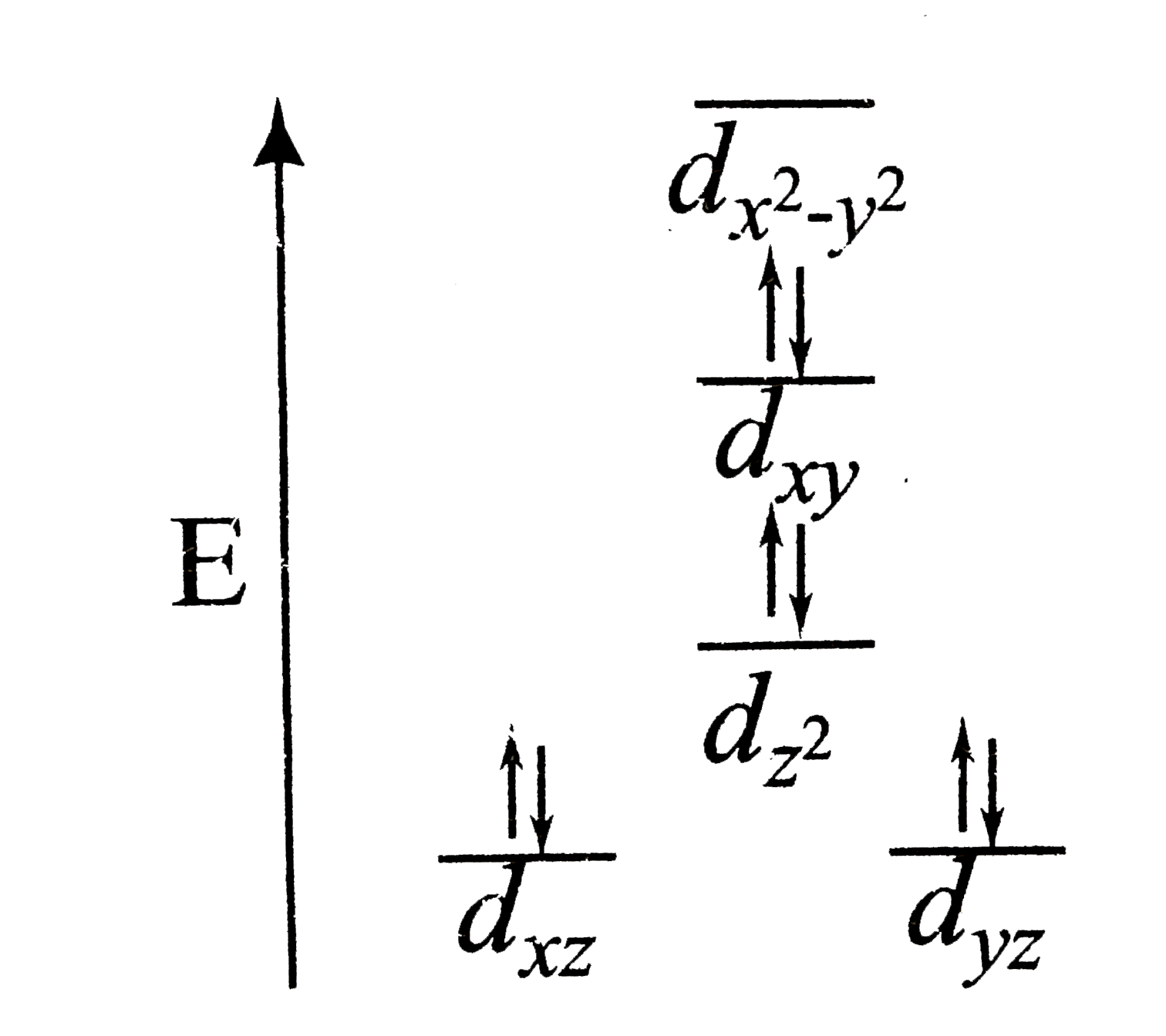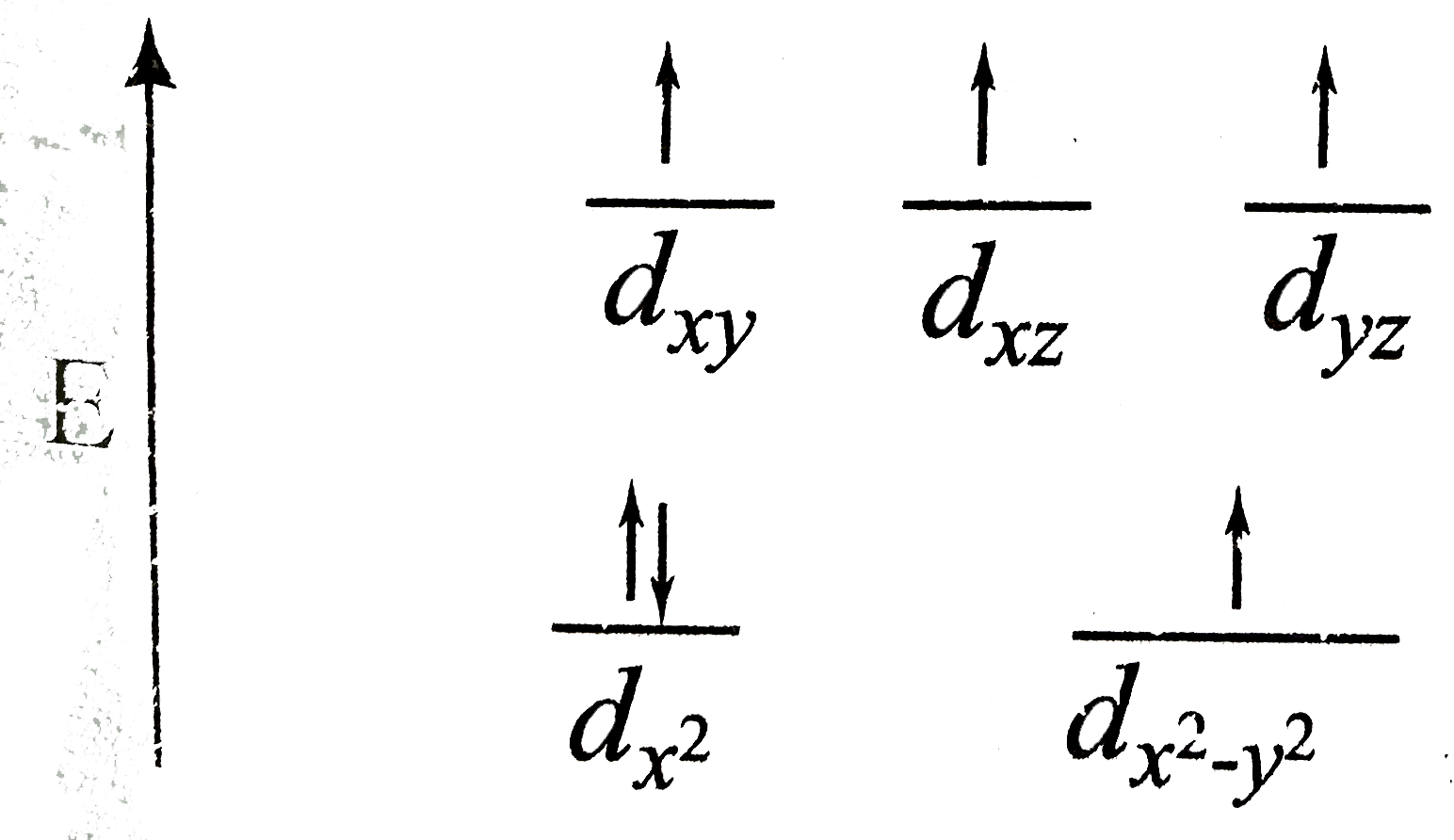A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-COORDINATION COMPOUNDS-Question Bank (Building the knowledge)
- Tetrahedral complexes of the types of [MA(4)] and [MA(3)B] (where M = ...
Text Solution
|
- The number of unpaired spins in the [Cr(en)(3)]^(2+) ion is
Text Solution
|
- Which of the following is paramagnetic and squre planar ?
Text Solution
|
- The magnitude of crystal field stabilization energy (CFSE) in tetrahed...
Text Solution
|
- A coordination compound of cobalt has the molecular, formula containin...
Text Solution
|
- IUPAC name of [Pt(NH(3))(3) (Br)(NO(2))CI] CI is
Text Solution
|
- The number of geometrical isomers of the complex [Co(NO(2))(3) (NH(3))...
Text Solution
|
- The formula of dichlorobis (urea) copper (II) is
Text Solution
|
- The complex ion [Co(NH(3))(6)]^(3+) is formed by sp^(3) d^(2) hybridir...
Text Solution
|
- Consider the follwing complexes ion P,Q and R P =[FeF(6)]^(3-), Q=[V...
Text Solution
|
- The number of geometric isomers that can exist for square planner comp...
Text Solution
|
- If the freezing point of a 0.01 molal aqueous solution of a cobalt (II...
Text Solution
|
- In the complex ion [Fe(H(2)O)(5)NO]^(2+)
Text Solution
|
- Which of the following statements is incorrect regarding the stereisom...
Text Solution
|
- Which of the following coordination entities are more stable for stron...
Text Solution
|
- The total number possible isomers for the complex compound [Cu^(II)(NH...
Text Solution
|
- Which of the folloiwng complexes in not expected to exhibit optical is...
Text Solution
|
- The octahedral complex of a metal ion M^(3+) with four monodentate lig...
Text Solution
|
- The equation which is balanced and represents the correct product(s) i...
Text Solution
|
- In the complex acetylbromidodicarbonyl bis (triethyphospine) iron (II)...
Text Solution
|