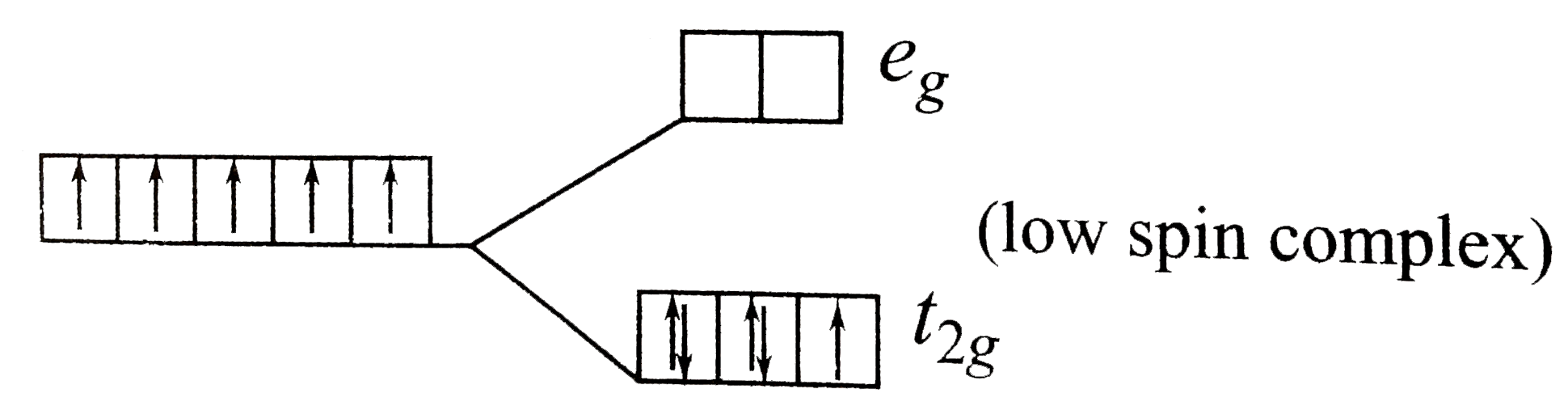
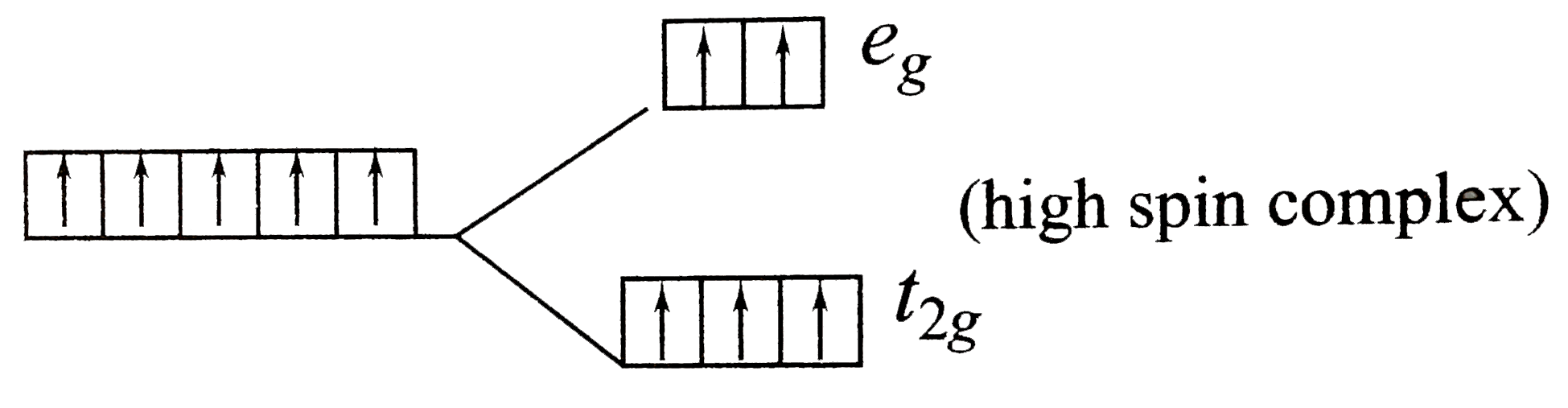
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-COORDINATION COMPOUNDS-Question Bank (Building the knowledge)
- The number of geometric isomers that can exist for square planner comp...
Text Solution
|
- If the freezing point of a 0.01 molal aqueous solution of a cobalt (II...
Text Solution
|
- In the complex ion [Fe(H(2)O)(5)NO]^(2+)
Text Solution
|
- Which of the following statements is incorrect regarding the stereisom...
Text Solution
|
- Which of the following coordination entities are more stable for stron...
Text Solution
|
- The total number possible isomers for the complex compound [Cu^(II)(NH...
Text Solution
|
- Which of the folloiwng complexes in not expected to exhibit optical is...
Text Solution
|
- The octahedral complex of a metal ion M^(3+) with four monodentate lig...
Text Solution
|
- The equation which is balanced and represents the correct product(s) i...
Text Solution
|
- In the complex acetylbromidodicarbonyl bis (triethyphospine) iron (II)...
Text Solution
|
- Among the complex ions, [Co(en)(2)CI(2)]^(+) , [CrCI(2)(C(2)O(4))(2)...
Text Solution
|
- Which of the following is a bidentate ligand ? (i) Acetylacetonate ...
Text Solution
|
- The IUPAC name of the complex [Cu(C(5)H(7)O(2))(2)] formed bby the rea...
Text Solution
|
- The IUPAC name for [(NH(3))(5)Cr - (OH) - Cr(NH(3))(5)] is
Text Solution
|
- The pair(s) of coordination complexes/ion exhibiting the same kind of ...
Text Solution
|
- EDTA^(4-) i9s ethylenediamine tetraacetate ion The total number of N-C...
Text Solution
|
- Which of the following compounds is not yellow coloured ?
Text Solution
|
- For the octahedral complex of Fe^(+) in SCN^(-) (thiocyanato-S) and in...
Text Solution
|
- An aqueous solution of metal ion MI reacts separately with reagents Q ...
Text Solution
|
- AIF(3) is soluble in HF only in presence of KF. It is due to the forma...
Text Solution
|