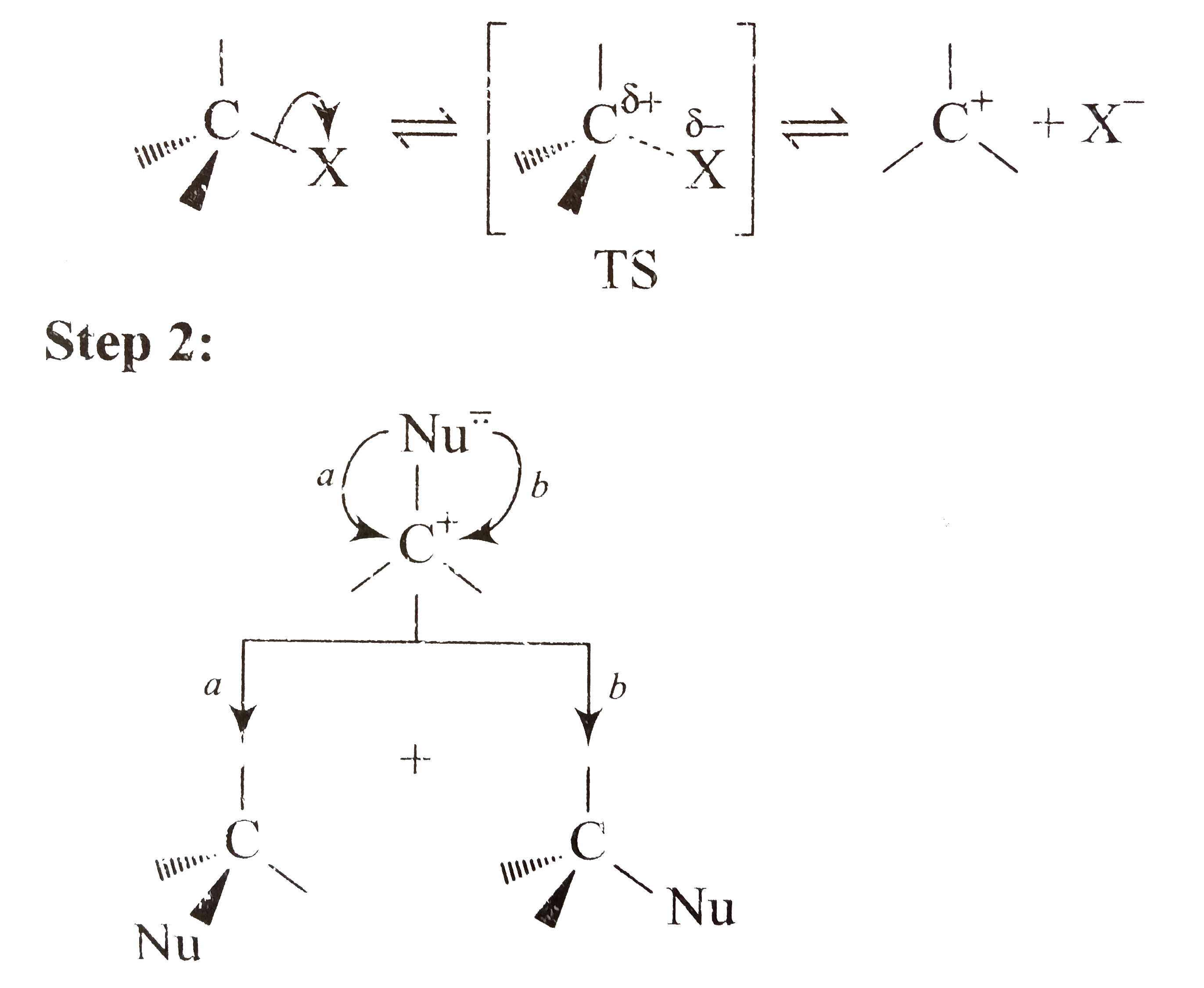
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-STEREOCHEMISTRY-Archives
- In which of the following moleucles, all atoms are coplanar?
Text Solution
|
- The correct corresponding order of names four aldoles with configurati...
Text Solution
|
- Which of the following bihphenyls is optically active?
Text Solution
|
- Two possible stereo-structures of CH(3)CHOHCOOH, which are optically a...
Text Solution
|
- In an S(N)1reaction on chiral centres, there is
Text Solution
|
- Which of the following compounds will undergo racemisation when soluti...
Text Solution
|
- Which of the following acids does not exhibit optical isomerism?
Text Solution
|
- How many steroisomers does this moelcules have? CH(3)CH=CHCH(2)CHBrC...
Text Solution
|
- If there is no rotation of plane polarized light by a compound in a sp...
Text Solution
|
- CH(3)-CHCI-CH(2)=CH(3) has a chiral centre. Which one of the following...
Text Solution
|
- Which of the following is not chiral?
Text Solution
|
- The chirality of the compound is
Text Solution
|
- Which of the following pairs of compounds are enantiomers?
Text Solution
|
- Which one of the following pairs represnets steroeoisomeris?
Text Solution
|
- In the reaction CH(3)CHO+HCNrarrCH(3)CH(OH)CNoverset(H(2)O)rarrCH(...
Text Solution
|
- A compound with molecular formula C(7)H(16) shows optical isomerism, t...
Text Solution
|
- R and S paris of enantiomers differ form one another in
Text Solution
|