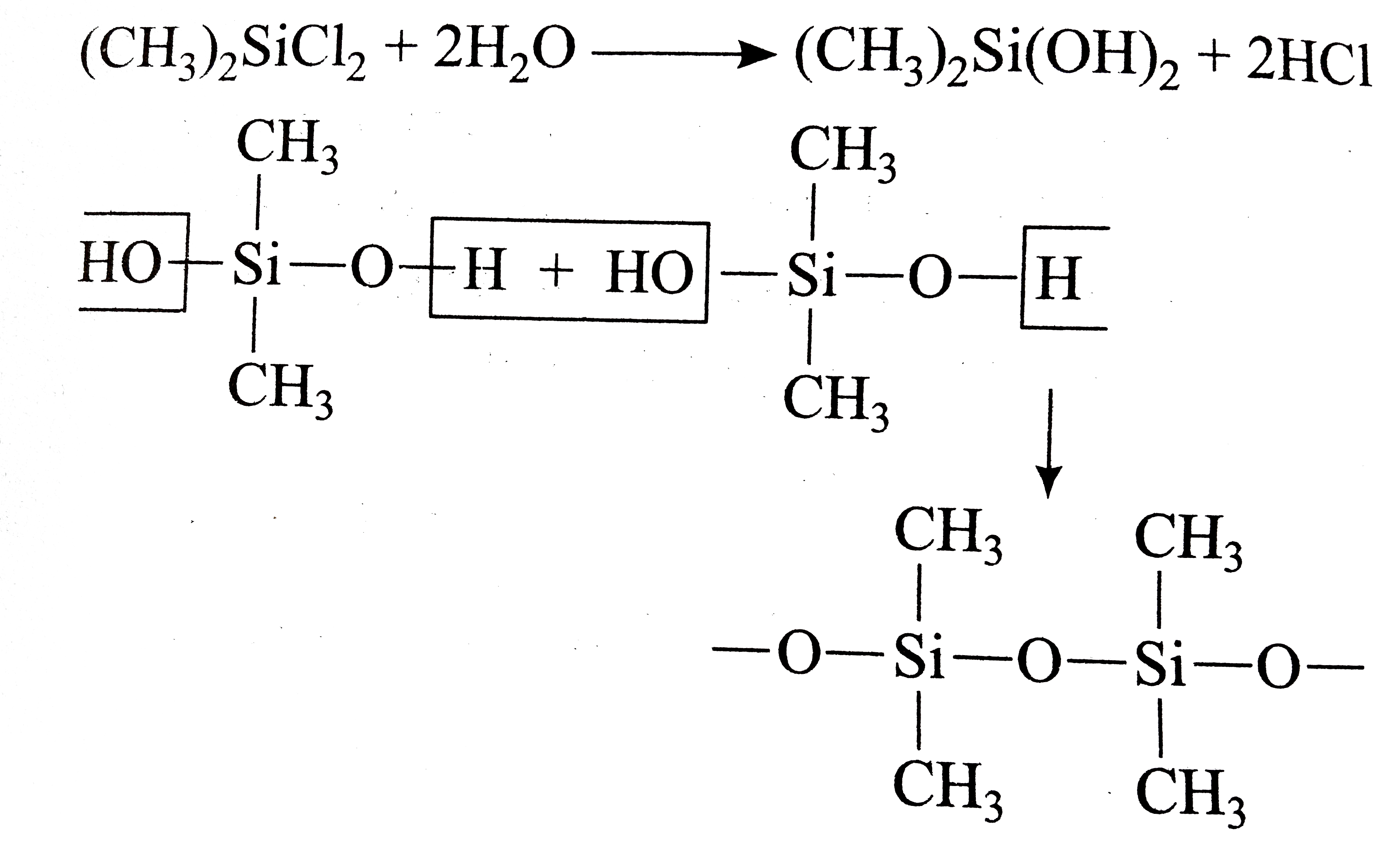A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-POLYMERS-All Questions
- Which is the monomer of neoprene in the following?
Text Solution
|
- Nylon is an example of
Text Solution
|
- Which one of the following is not a condensation polymer?
Text Solution
|
- Which of the following statements is false?
Text Solution
|
- Which one of the following sets forms the biodegrable polymer?
Text Solution
|
- Of the following which one is classified as polyester polymer?
Text Solution
|
- Which of the following structures represents neoprene polymers?
Text Solution
|
- Struchures of some common polymers are given. Which one is not correct...
Text Solution
|
- The straight chain polymer is formed by
Text Solution
|
- Which of the following statement is not ture?
Text Solution
|
- Which one of the following polymers is prepared by condensation polyme...
Text Solution
|
- ~[NH (CH(2)) NHCO (CH(2))(4) CO]~ is a
Text Solution
|
- The monomer of the polymer ~CH(2)-underset(CH(3))underset(|)overset(...
Text Solution
|
- Which one of the following is a chain growth polymer?
Text Solution
|
- Which one of the following monomers gives the polymer neoprene on poly...
Text Solution
|
- Acrilan is a hard, horny and a high melting matrial. Which of the foll...
Text Solution
|
- Cellulose is a polymer of
Text Solution
|
- Monomer of [-overset(CH(3))overset(|)underset(CH(3))underset(|)(C)-C...
Text Solution
|
- Which one of the following is not correctly matched?
Text Solution
|
- CF(2) = CF(2) is a monomer of
Text Solution
|