Similar Questions
Explore conceptually related problems
Recommended Questions
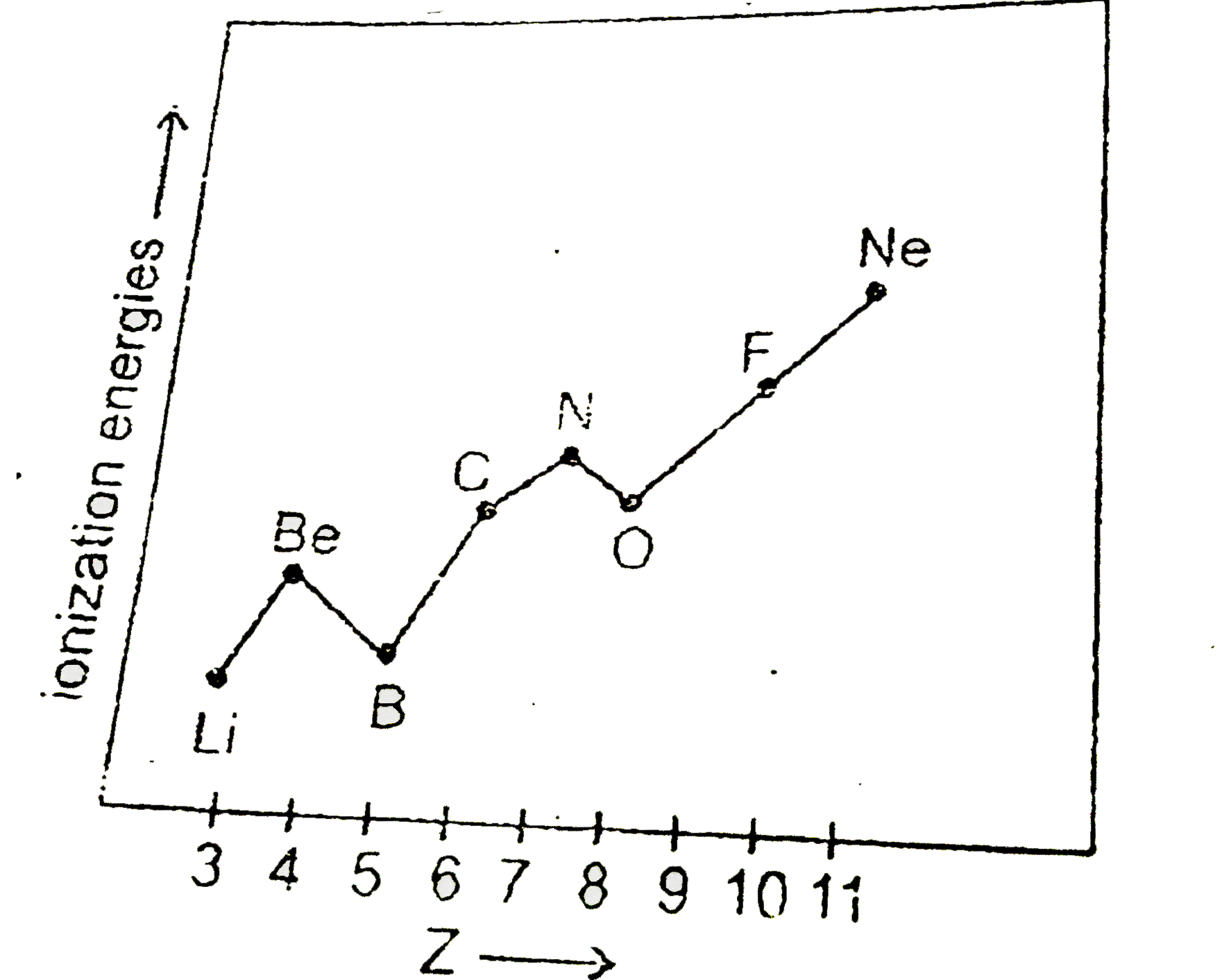
- Following graph shows variation of ionization energies with atomis num...
Text Solution
|
- The second ionization energies of Li, Be, B and C are in the order
Text Solution
|
- the ionization energy of Li ^(++) is equal to
Text Solution
|
- Following graph shows variation of ionization energies with atomis num...
Text Solution
|
- बेरेलियम की आयनन ऊर्जा Li तथा B की आयनन ऊर्जा की अपेक्षा अधिक क्यों हो...
Text Solution
|
- The second ionization energies of Li ,Be , B and C are in the order
Text Solution
|
- Li , Be, B, Na को बढ़ती आयनन ऊर्जा के कर्म में व्यवस्थित करें।
Text Solution
|
- (a) Define ionization energy. (b) Prove that ionization energy is a ...
Text Solution
|
- Na^(+),Ne లకు ఒకే ఎలక్ట్రాన్ విన్యాసం ఉన్నప్పటికీ, Na^(+) కు Ne కంటే ఎ...
Text Solution
|