Similar Questions
Explore conceptually related problems
Recommended Questions
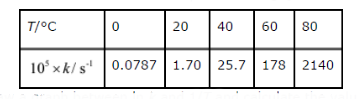
- The rate constant for the decomposition of N(2)O(5) at various tempera...
Text Solution
|
- Graph between log k and 1//T [k rate constant (s^(-1)) and T and the t...
Text Solution
|
- The experimental data for decomposition of N(2)O(5) [2N(2)O(5)to 4N...
Text Solution
|
- The rate constant for the decomposition of N(2)O(5) at various tempera...
Text Solution
|
- The experiment data for decomposition of N(2)O(5)[2N(2)O(5) to 4NO(2) ...
Text Solution
|
- The rate constant for the decomposition of N(2)O(5) at various tempera...
Text Solution
|
- Graph between log k and 1//T [where K is rate constant in s^(-1) and T...
Text Solution
|
- The graph between log k and 1/T[K is rate constant (sec^(-1)) and T th...
Text Solution
|
- गैस प्रावस्था में 318 K पर N(2)O(5) के अपघटन की [2N(2)O(5)rarr4NO(2)+O...
Text Solution
|