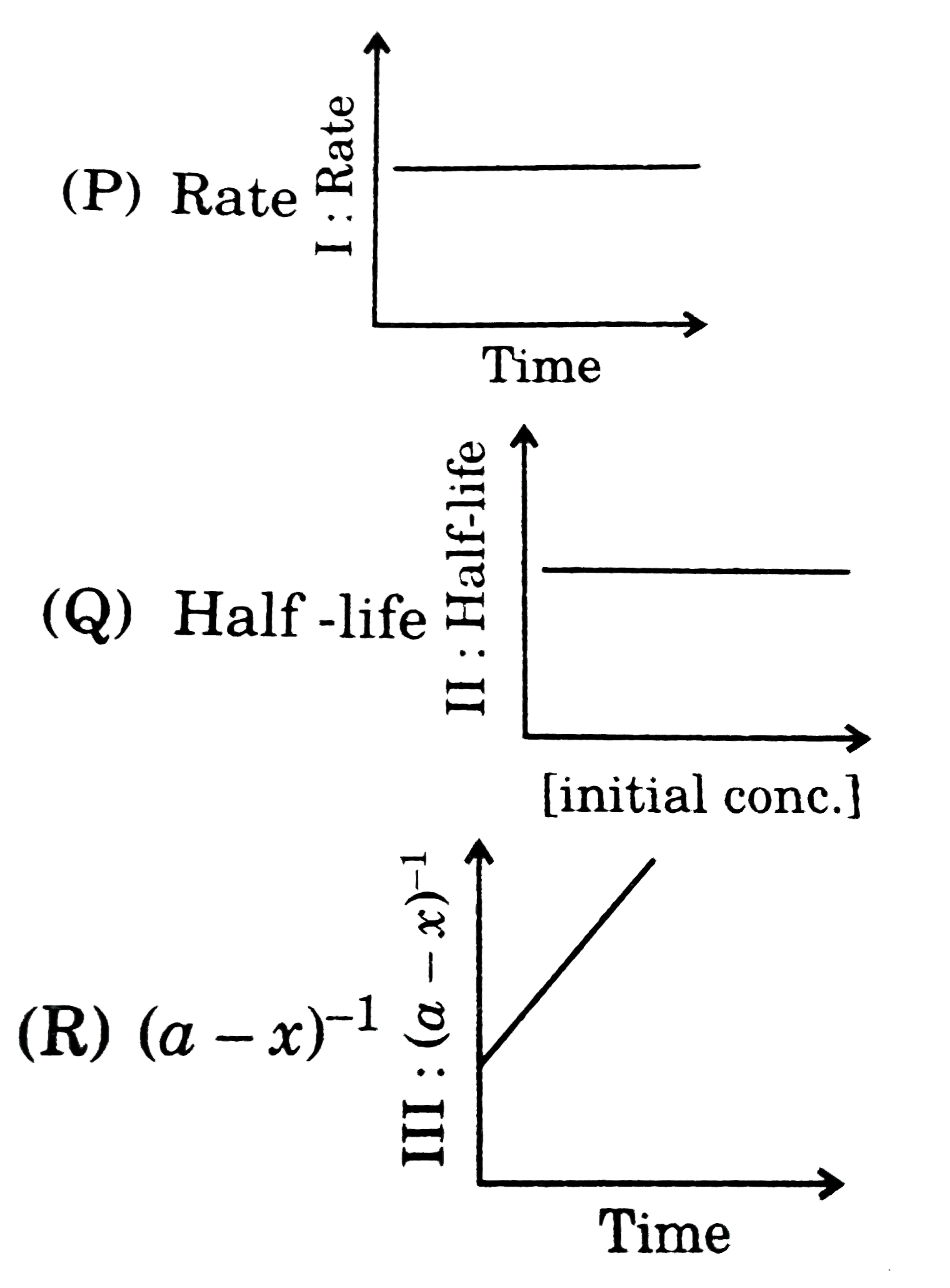Similar Questions
Explore conceptually related problems
Recommended Questions
- Match the graphical study with the order of the reaction: A: Firs...
Text Solution
|
- Match the graphical study with the order of the reaction: A: First, B:...
Text Solution
|
- Write the rate law for the following reactions (a) A reactions that is...
Text Solution
|
- Give the units of rate constants for Zero, first order and second orde...
Text Solution
|
- अभिक्रिया की कोटि क्या है? शून्य कोटि, प्रथम कोटि एवं द्वितीय कोटि की ...
Text Solution
|
- What is order of reaction ? Write units of the rate constant k for zer...
Text Solution
|
- अभिक्रिया की कोटि क्या है ? शून्य कोटि, प्रथम कोटि एवं द्वितीय कोटि की...
Text Solution
|
- 3A+2B+CrarrD+E is a first order reaction with respect to A , second or...
Text Solution
|
- Graphical representation of zero and first order of kinetics| first or...
Text Solution
|