Similar Questions
Explore conceptually related problems
Recommended Questions
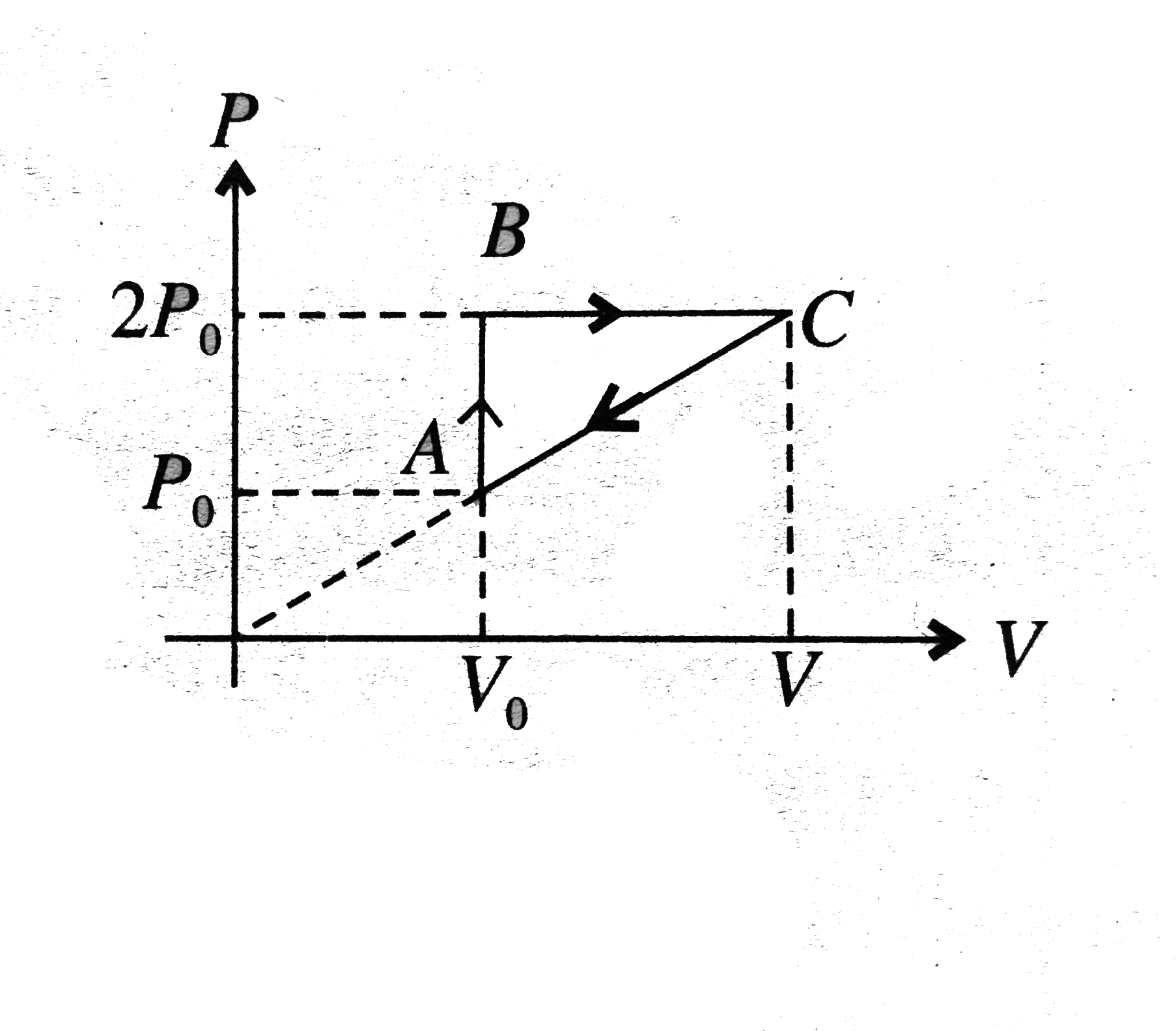
- n moles of a diatomic gas has undergone a cyclic process ABC as shown ...
Text Solution
|
- The density (rho) versus pressure (p) graph of one mole of an ideal mo...
Text Solution
|
- n moles of a diatomic gas has undergone a cyclic process ABC as shown ...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- In the figure n mole of a monoatomic ideal gas undergo the process ABC...
Text Solution
|
- For an ideal gas the ratio of specific heats is (C(p))/(C(v)) = gamma ...
Text Solution
|
- One mole of ideal monatomic gas in taken in cyclice process ABCA a...
Text Solution
|
- An ideal gas follows a cyclic process as shown in figure. Internal ene...
Text Solution
|