Similar Questions
Explore conceptually related problems
Recommended Questions
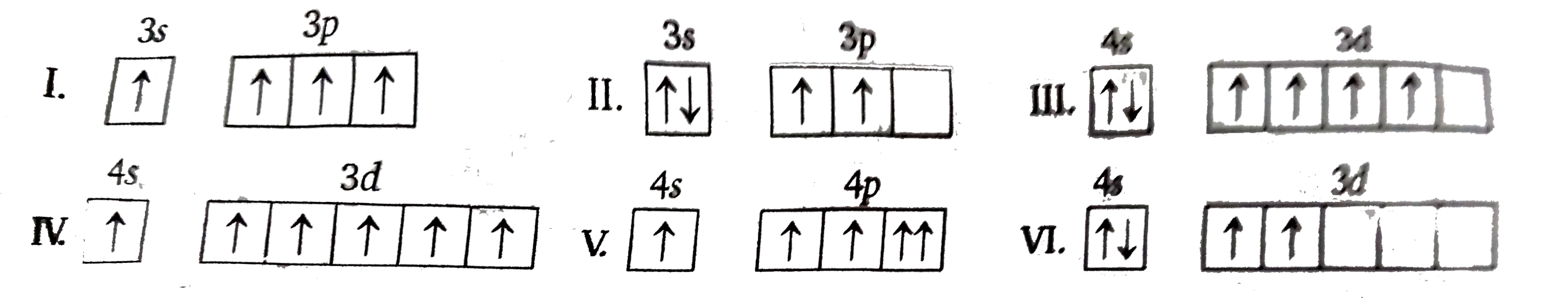
- Consider the following six electronic configurations (remaining inner ...
Text Solution
|
- The inner shells of electrons are completely filled in
Text Solution
|
- In the following six electronic configuration (remaining inner orbital...
Text Solution
|
- Consider the following six electronic configurations (remaining inner ...
Text Solution
|
- Electron configurations of few elements are given below,Mark the incor...
Text Solution
|
- Give the outer orbit general electronic configuration of (d) Inner t...
Text Solution
|
- Electronic configuration of few elements are given below. Mark the inc...
Text Solution
|
- किसी तत्व के इलेक्ट्रॉनिक विन्यास में np^(6) के बाद निम्न कक्षक भरा जा...
Text Solution
|
- Which of the following electronic configuration is incorrect ?
Text Solution
|