Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CALORIMETRY-Level- II (H.W)
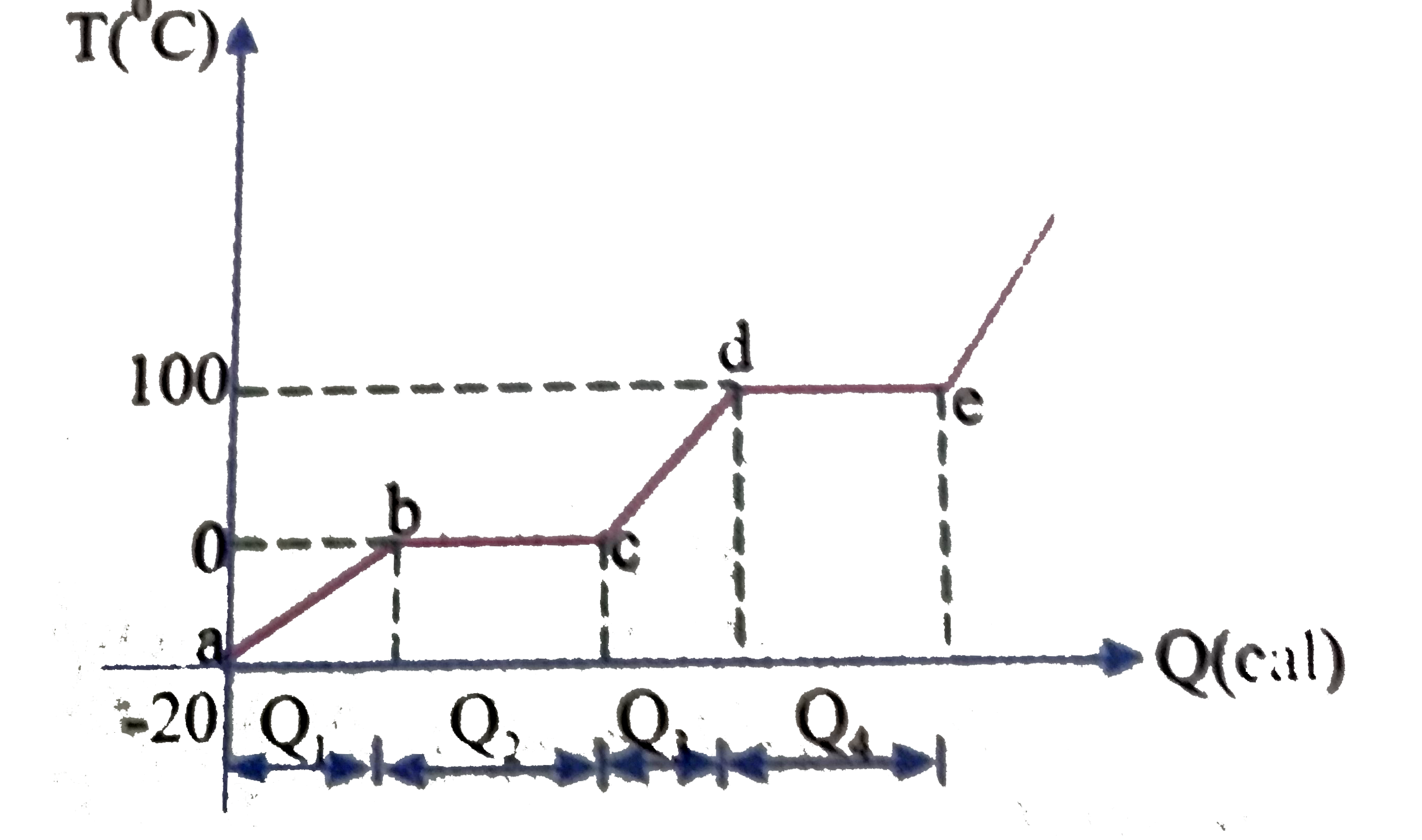
- The following graph represents change of state of 1 gram of ice at -20...
Text Solution
|
- A calorimeter takes 200 cal of heat to rise its temperature through 10...
Text Solution
|
- Three different substances have the specific heats in the ratio 1:2 : ...
Text Solution
|
- Equal masses of 3 liquids A,B and C have temperatures 10^@C, 25^@C and...
Text Solution
|
- 1 gram of ice at -10^@ C is converted to steam at 100^@ C the amount o...
Text Solution
|
- 30 gram copper is heated to increase its temperature by 20^@ C if the ...
Text Solution
|
- A liquid of mass m and specific heat c is heated to a temperature 2T. ...
Text Solution
|
- A tap supplies water at 10^@ C and another tap at 100^@ C. How much ho...
Text Solution
|
- The amount of heat supplied to decrease the volume of an ice water mix...
Text Solution
|
- The power of a system which can convert 10 kg of water at 30^@ C into ...
Text Solution
|
- The amount of steam at 100^@ C that should be passed into 600 g of wat...
Text Solution
|
- M' kg of water 't' 0^@ C is divided into two parts so that one part of...
Text Solution
|
 .
.