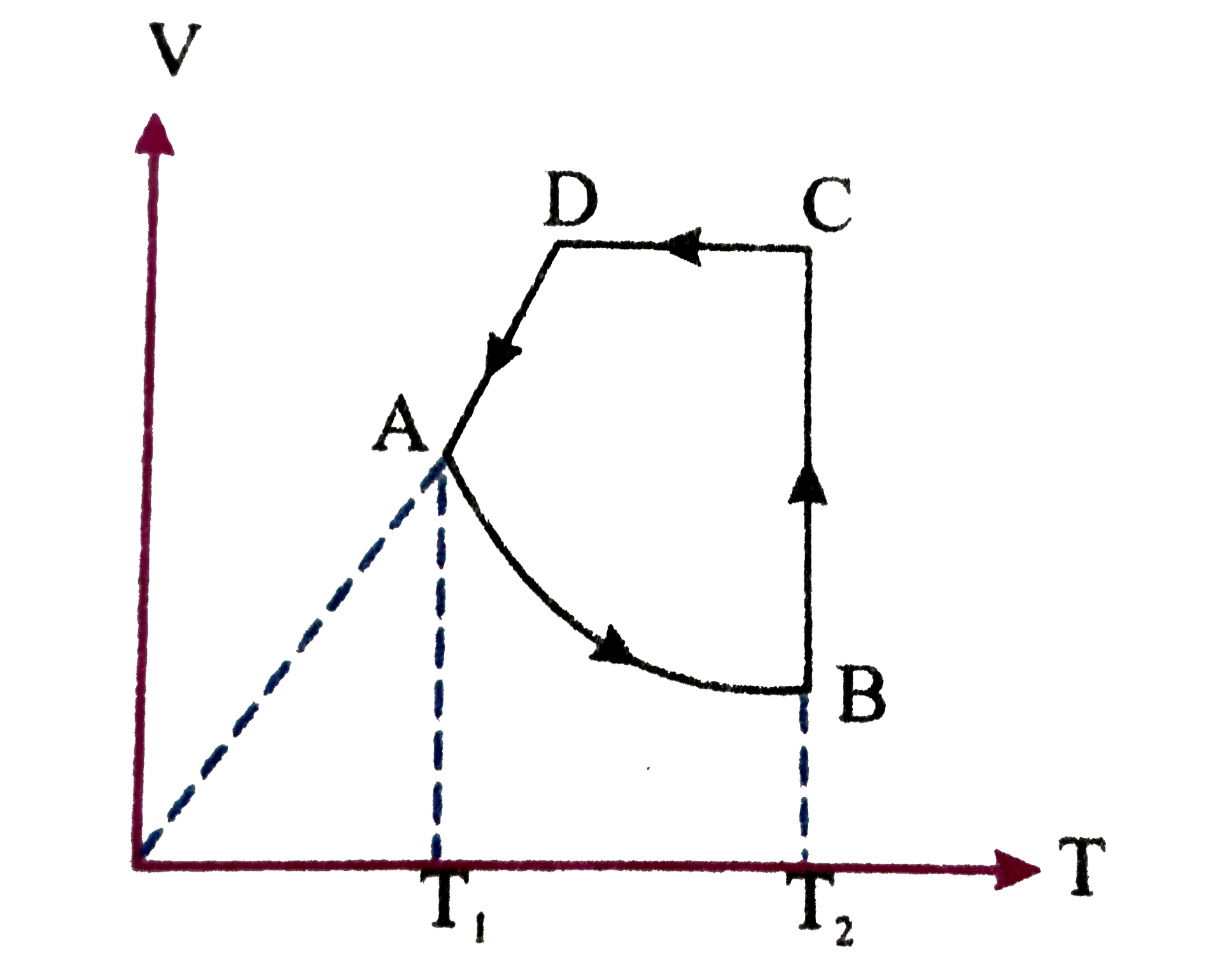
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Exercise
- An amount Q of heat is added to a mono atomic ideal gas in a process i...
Text Solution
|
- Figure shows a cycle ABCDA undergone by 2 moles of an ideal diatomic g...
Text Solution
|
- One mole of an ideal monoatmic gas undergoes a process defined y U=asq...
Text Solution
|
- An ideal gas can be expanded from an initial state to a certain volume...
Text Solution
|
- Monoatomic , diatomic and triatomic gases whose initial volume and pre...
Text Solution
|
- A cyclic process ABCD is shown is shown in the following P-V diagram. ...
Text Solution
|
- A cyclic process is shown in the P-T diagram. Which of the curves show...
Text Solution
|
- An ideal monoatomic gas is taken the cycle ABCDA as shown in following...
Text Solution
|
- Ideal gas is taken through the process shown in the figure :
Text Solution
|
- The specific heat of solids at low temperatures varies with absolute t...
Text Solution
|
- In the P-V diagram shown shown in figure ABC is a semicircle.The work ...
Text Solution
|
- A thermodynamic system undergoes cyclic process ABCDA as shown in figu...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adial gas is a...
Text Solution
|
- The figure shows two paths for the change of state of a gas from A to ...
Text Solution
|
- 3 moles of an ideal mono atomic gas performs a cycle as shown in fig. ...
Text Solution
|
- A metal block of density 5000kg//m^(3) and mass 2kg in suspended by a ...
Text Solution
|
- One mole of Argon undergoes a process given by PV^(3//2)= const. If he...
Text Solution
|
- 2kg of ice at 20^@C is mixed with 5kg of water at 20^@C in an insulati...
Text Solution
|
- Steam at 100^@C is passed into 1.1 kg of water contained in a calorime...
Text Solution
|
- A block of ice at -10^@C is slowly heated and converted to steam at 10...
Text Solution
|