A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Exercise
- One mole of an ideal monoatomatic gas is taken round the cylic process...
Text Solution
|
- Molar heat capacity of an ideal gas varies as C = C(v) +alphaT,C=C(v)+...
Text Solution
|
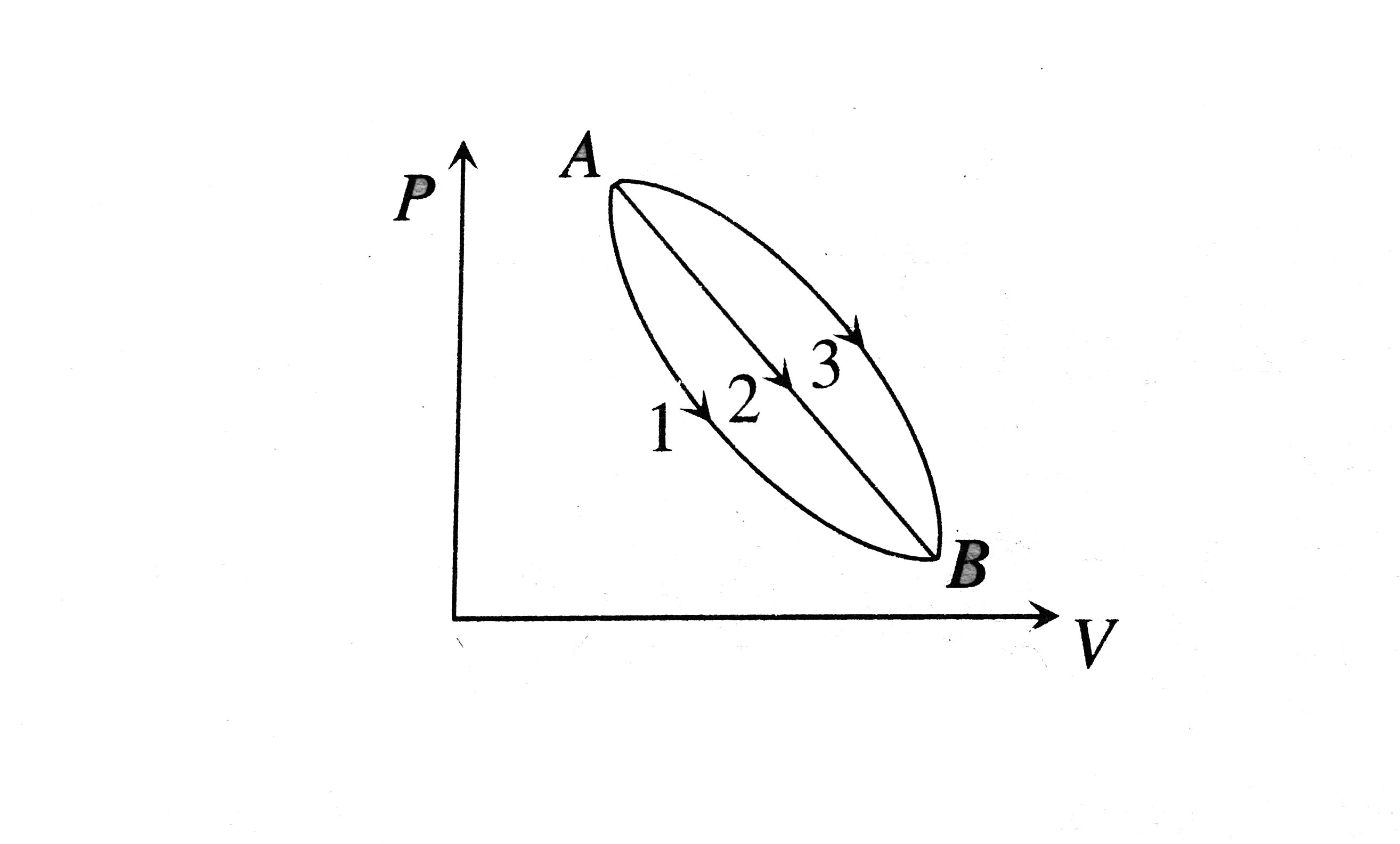
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- An ideal gas undergoes an expansion from a state with temperature T(1)...
Text Solution
|
- Select the correct alternatives for an ideal gas :
Text Solution
|
- P - V diagram of a cyclic process ABCA is as shown in Fig. Choose the ...
Text Solution
|
- Figure shows the P-V siagram of a cyclic process. If dQ is the heat en...
Text Solution
|
- Two gases have the same initial pressure, volume and temperature. They...
Text Solution
|
- During the process AB of an ideal gas
Text Solution
|
- 1 kg of ice at 0^(@)C is mixed with 1.5 kg of water at 45^(@)C [latent...
Text Solution
|
- In a thermodynamic process helium gas obeys the law TP^(2//5) = consta...
Text Solution
|
- A gas is found to obey the law P^(2)V= constant. The initial temperatu...
Text Solution
|
- The figure shows the P-V plot of an ideal gas taken through a cycle AB...
Text Solution
|
- Cv and Cp denote the molar specific heat capacities of a gas at consta...
Text Solution
|
- An ideal gas is taken from the state A (pressure p, volume V) to the s...
Text Solution
|
- During the melting of a slab of ice at 273K at atmospheric pressure,
Text Solution
|
- One mole of an ideal gas in initial state A undergoes a cyclic process...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|