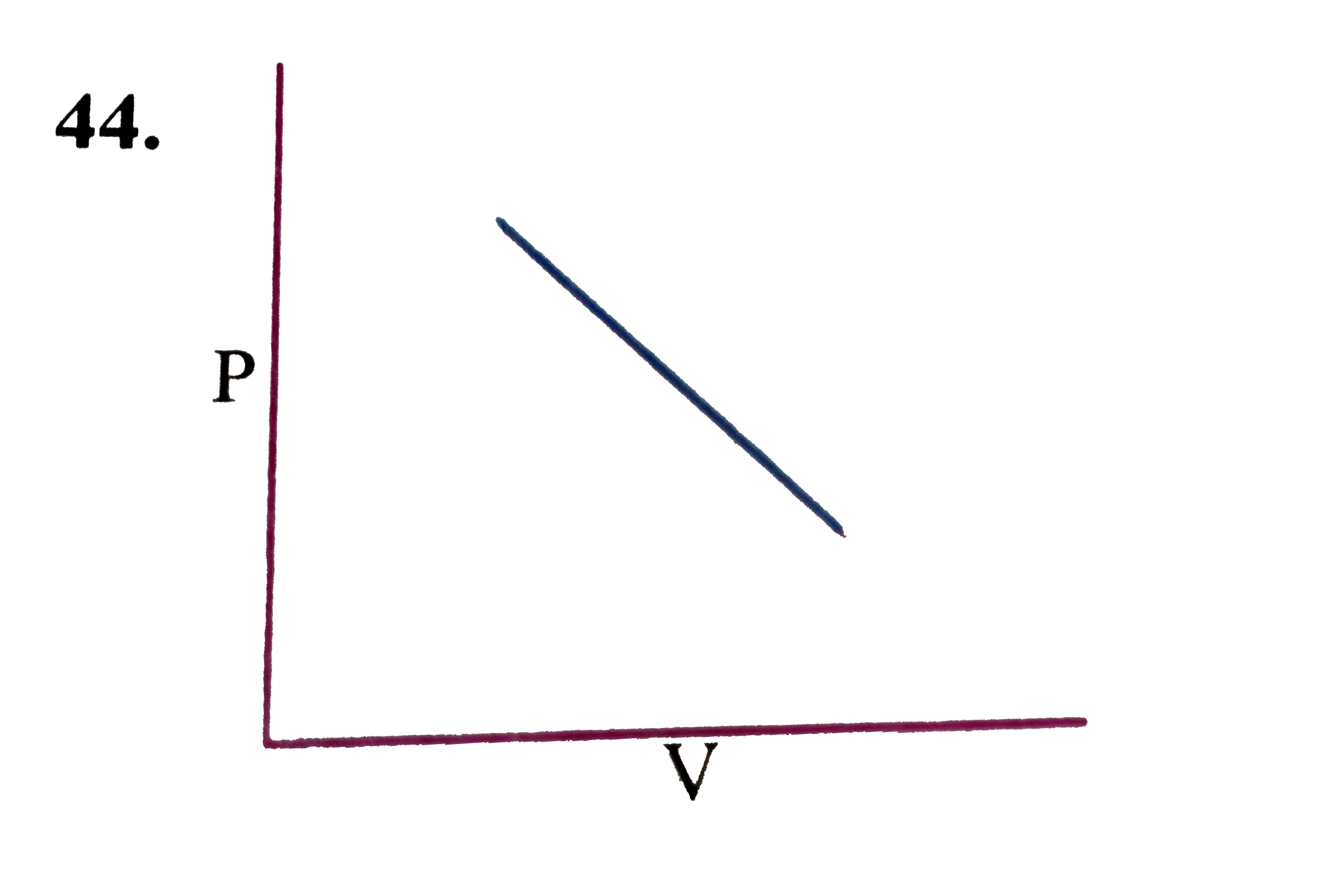
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Exercise
- The figure shows the P-V plot of an ideal gas taken through a cycle AB...
Text Solution
|
- Cv and Cp denote the molar specific heat capacities of a gas at consta...
Text Solution
|
- An ideal gas is taken from the state A (pressure p, volume V) to the s...
Text Solution
|
- During the melting of a slab of ice at 273K at atmospheric pressure,
Text Solution
|
- One mole of an ideal gas in initial state A undergoes a cyclic process...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A calorimeter of mass m contains an equal mass of water in it. The tem...
Text Solution
|
- A calorimeter of mass m contains an equal mass of water in it. The tem...
Text Solution
|
- A calorimeter of mass m contains an equal mass of water in it. The tem...
Text Solution
|
- Two mole of an ideal monatomic gas are confined within a cylinder by a...
Text Solution
|
- Two mole of an ideal monatomic gas are confined within a cylinder by a...
Text Solution
|
- Two mole of an ideal monatomic gas are confined within a cylinder by a...
Text Solution
|
- A mercury barometer is defective. When an accurate barometer reads 770...
Text Solution
|
- A mercury barometer is defective. When an accurate barometer reads 770...
Text Solution
|
- A 50 gm lead bullet (sp. Heat 0.020 cal//g) is initially at 30^(@)C . ...
Text Solution
|
- An ideal gas (Cp / Cv = gamma) is taken through a process in which the...
Text Solution
|
- A Vessel contains helium, which expands at constant pressure when 15 k...
Text Solution
|
- When a quantity of liquid bismuth at its melting point is transferred ...
Text Solution
|