Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Exercise
- Figure shows the variation of internal energy (U) with the pressure (P...
Text Solution
|
- The heat absorbed by a system in going through the cyclic process show...
Text Solution
|
- 1//R(R"is universal gas constant") moles of an ideal gas (gamma=1.5) u...
Text Solution
|
- A metal rod AB of length 10x has its one end A in ice at 0^@C, and the...
Text Solution
|
- An ideal diatomic gas under goes a process in which its internal ener...
Text Solution
|
- An ideal gas is taken through a cyclic thermodynamic process through f...
Text Solution
|
- A piece of ice (heat capacity =2100 J kg^(-1) .^(@)C^(-1) and latent h...
Text Solution
|
- A diatomic ideal gas is compressed adiabatically to 1/32 of its initia...
Text Solution
|
- When a system is taken from state i to state f alone the path iaf, it ...
Text Solution
|
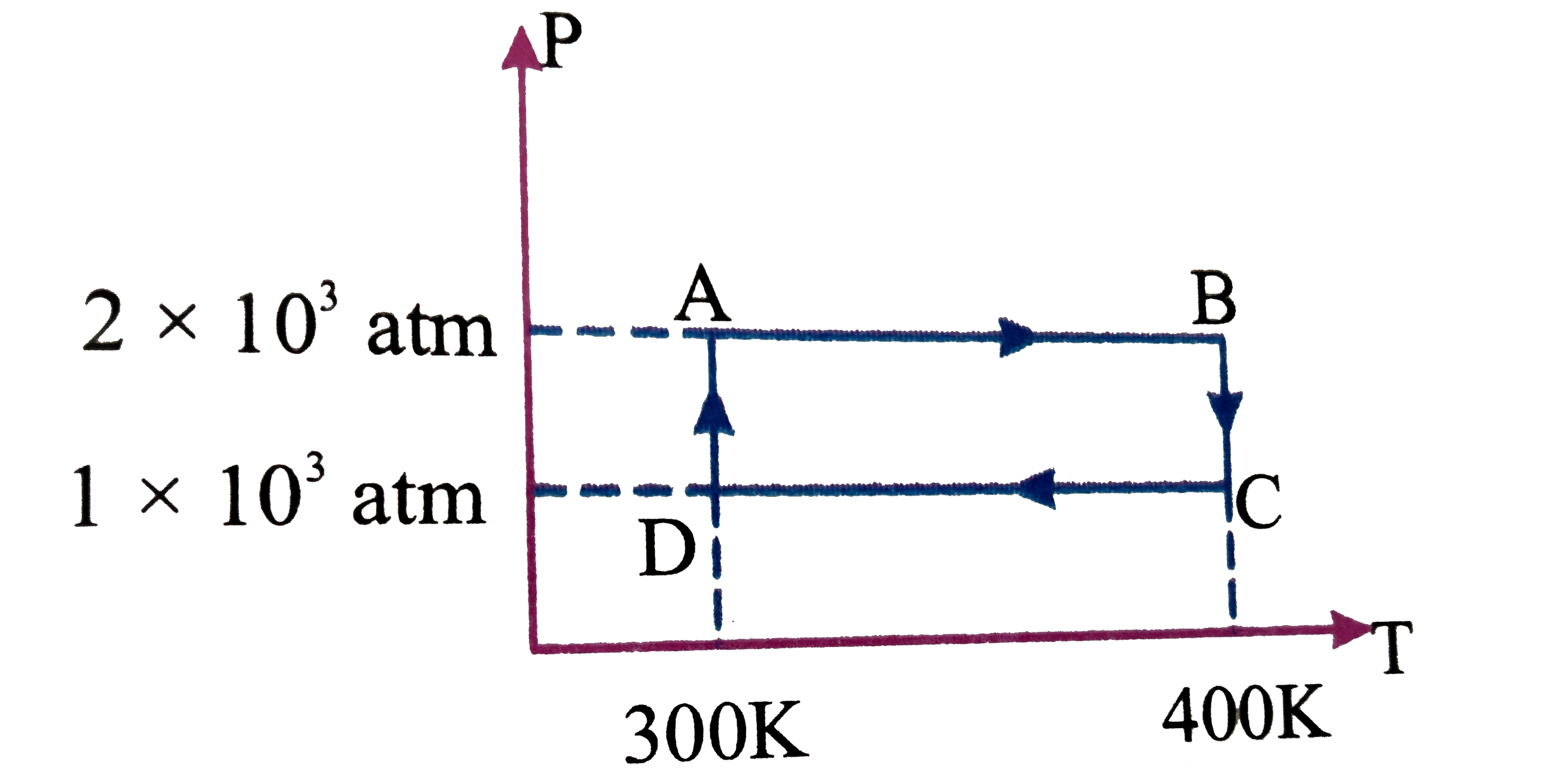
- Two moles of helium gas undergo a cyclic process as shown in Fig. Assu...
Text Solution
|
- In a insulated vessel, 0.05 kg steam at 373 K and 0.45 kg of ice at 25...
Text Solution
|
- An ice cube of mass 0.1 kg at 0^@C is placed in an isolated container ...
Text Solution
|
- A diatomic gas is enclosed in a vessel fitted with massless movable pi...
Text Solution
|
- Two moles of an ideal monoatomic gas is taken through a cycle ABCA as ...
Text Solution
|
- Molar specific heat at constant volume for an ideal gas is given by C(...
Text Solution
|
- An ice cube of mass M(0) is given a velocity v(0) on a round horizonta...
Text Solution
|
- Figure shows a cyclic process ABCDBEA performed on an ideal cycle. If ...
Text Solution
|
- Volume versus temperature graph of two moles of helium gas is as shown...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- A vessel of water eqivalent W kg contains m kg of water of specific he...
Text Solution
|