Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Exercise
- An ice cube of mass 0.1 kg at 0^@C is placed in an isolated container ...
Text Solution
|
- A diatomic gas is enclosed in a vessel fitted with massless movable pi...
Text Solution
|
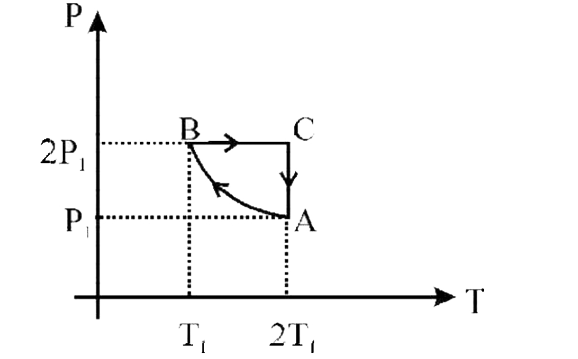
- Two moles of an ideal monoatomic gas is taken through a cycle ABCA as ...
Text Solution
|
- Molar specific heat at constant volume for an ideal gas is given by C(...
Text Solution
|
- An ice cube of mass M(0) is given a velocity v(0) on a round horizonta...
Text Solution
|
- Figure shows a cyclic process ABCDBEA performed on an ideal cycle. If ...
Text Solution
|
- Volume versus temperature graph of two moles of helium gas is as shown...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- A vessel of water eqivalent W kg contains m kg of water of specific he...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- The work done by a cretain material when temprature changes from T(0) ...
Text Solution
|
- An ideal gas is made to undergo a termodynamic process given by V prop...
Text Solution
|
- Determine the average molar heat capacity of an ideal gas under going ...
Text Solution
|
- An ideal gas goes through a cycle consisting of ischoric adiabatic and...
Text Solution
|
- A cycle is made of three process is iso barie, adiabatic and isotherma...
Text Solution
|
- A calorimeter contains 4.00 kg of water at 20.0^(@)C . What amount of ...
Text Solution
|
- A 0.50 kg ice cube at -10^(@)C is placed in 3.0 kg of coffce at 20^(@)...
Text Solution
|
- A thermal insulated vessel contains some water at 0^(@)C. The vessel i...
Text Solution
|
- If specific heat capacity of a substance in solid and liquid is propor...
Text Solution
|
- 450 g water at 40^(@)C was kept in a calorimeter of water equivalent 5...
Text Solution
|