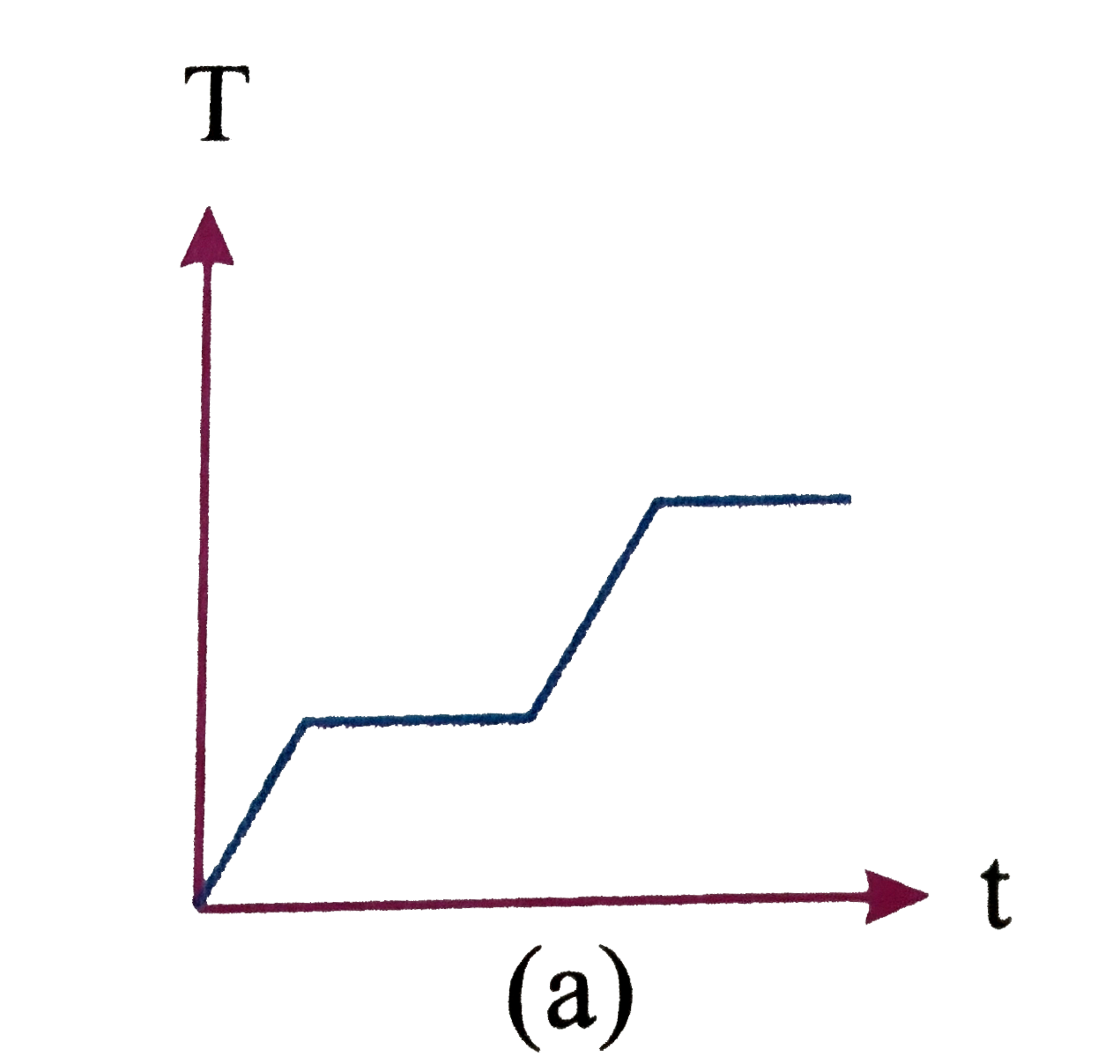
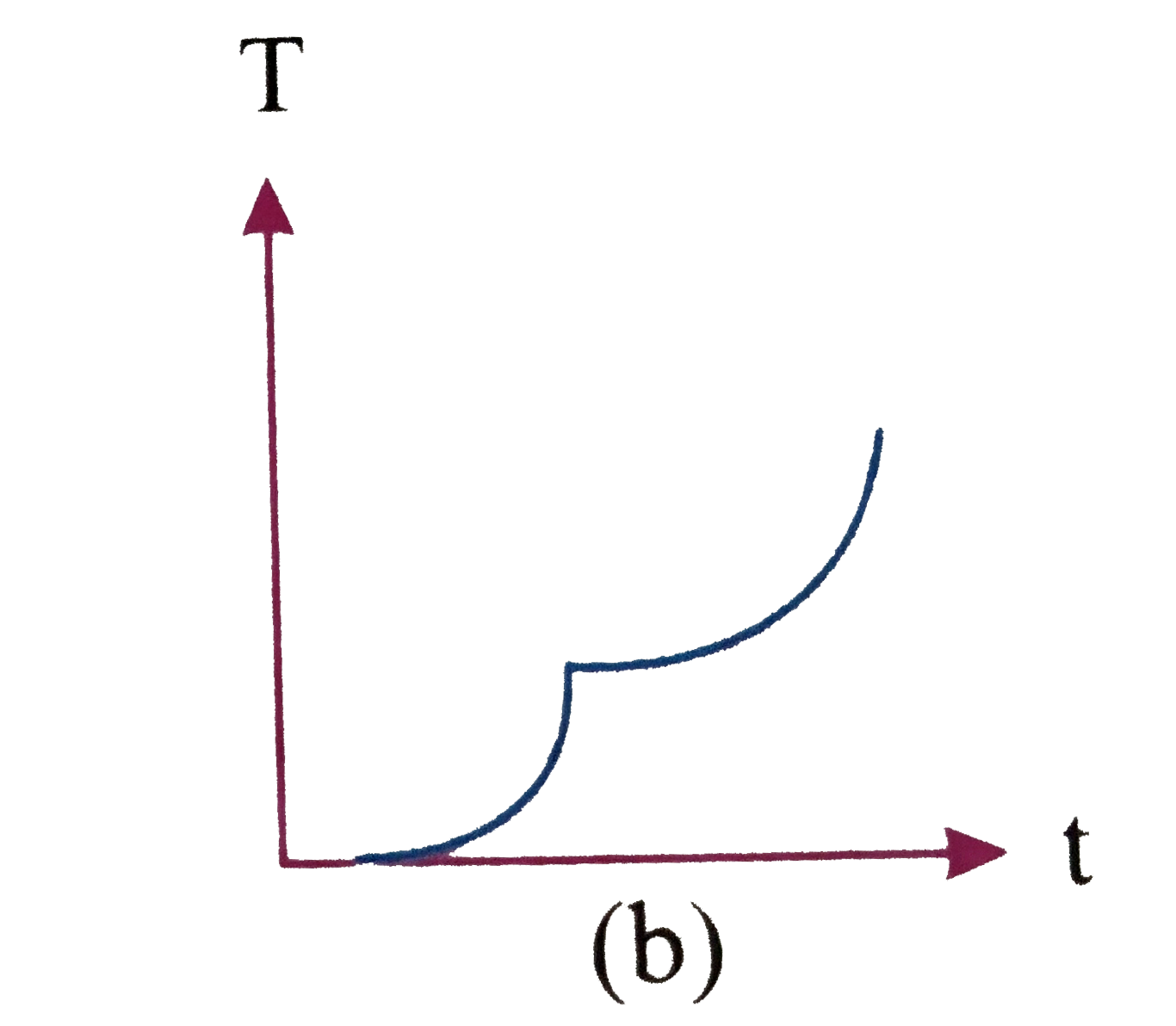
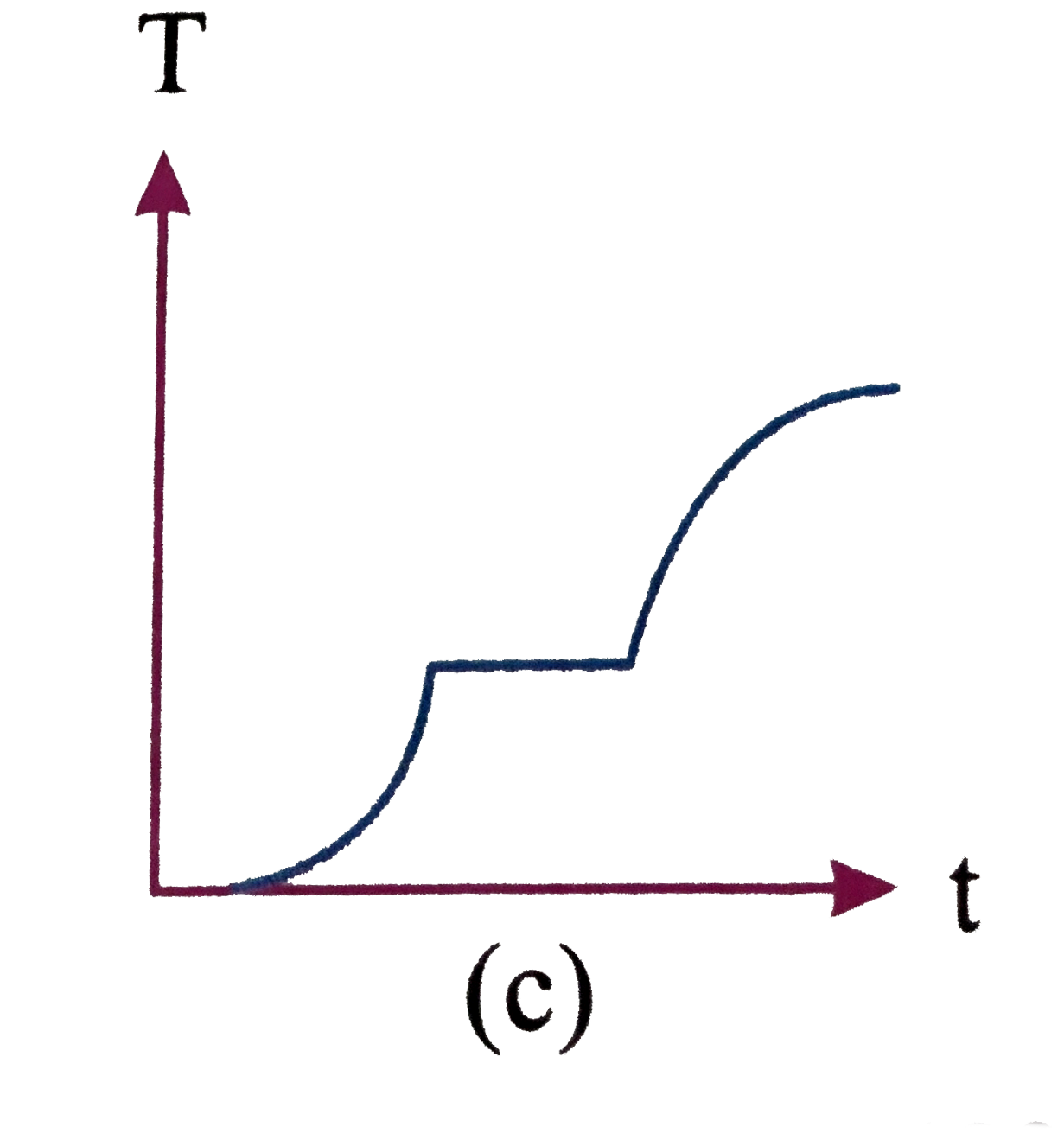
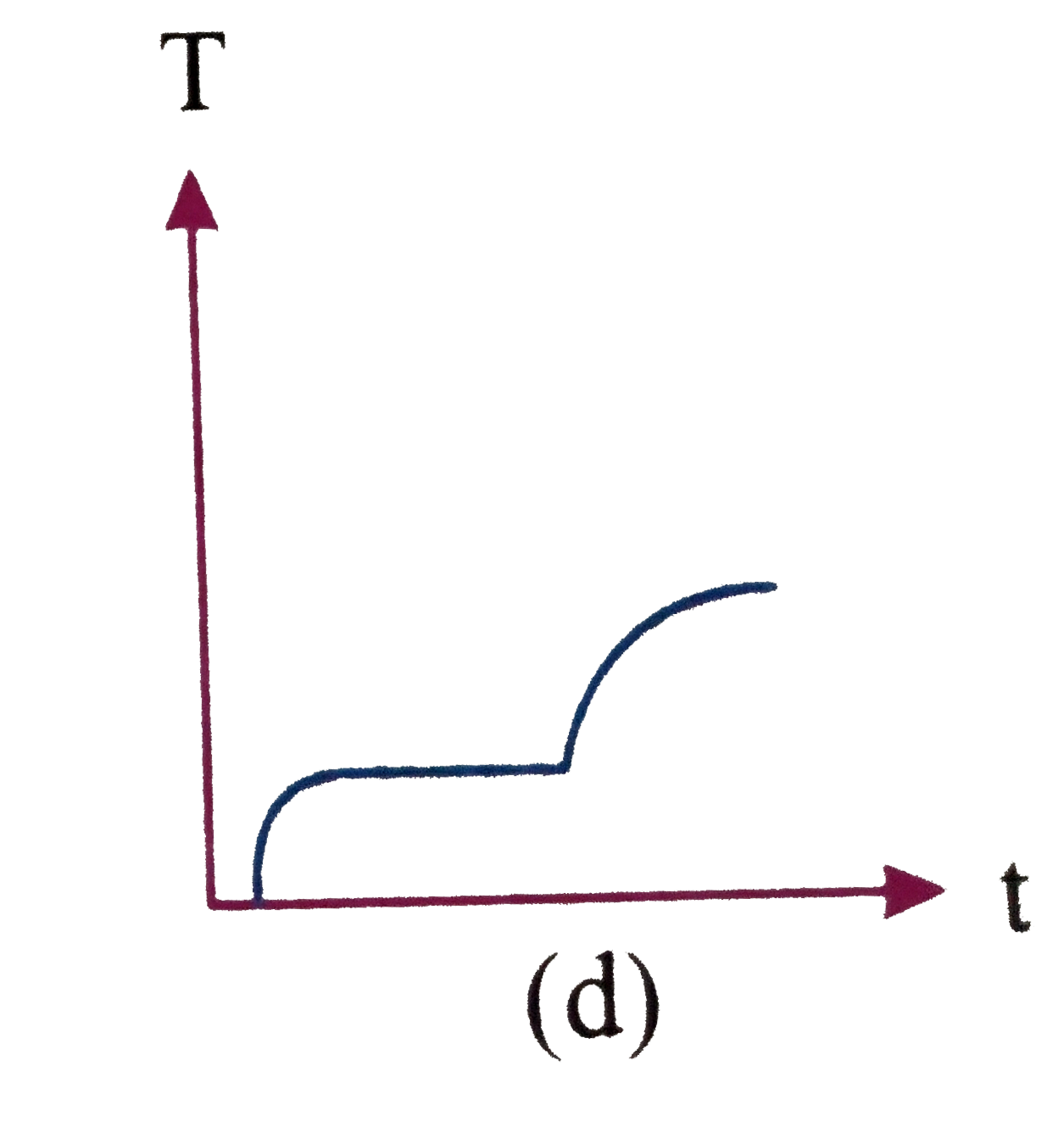
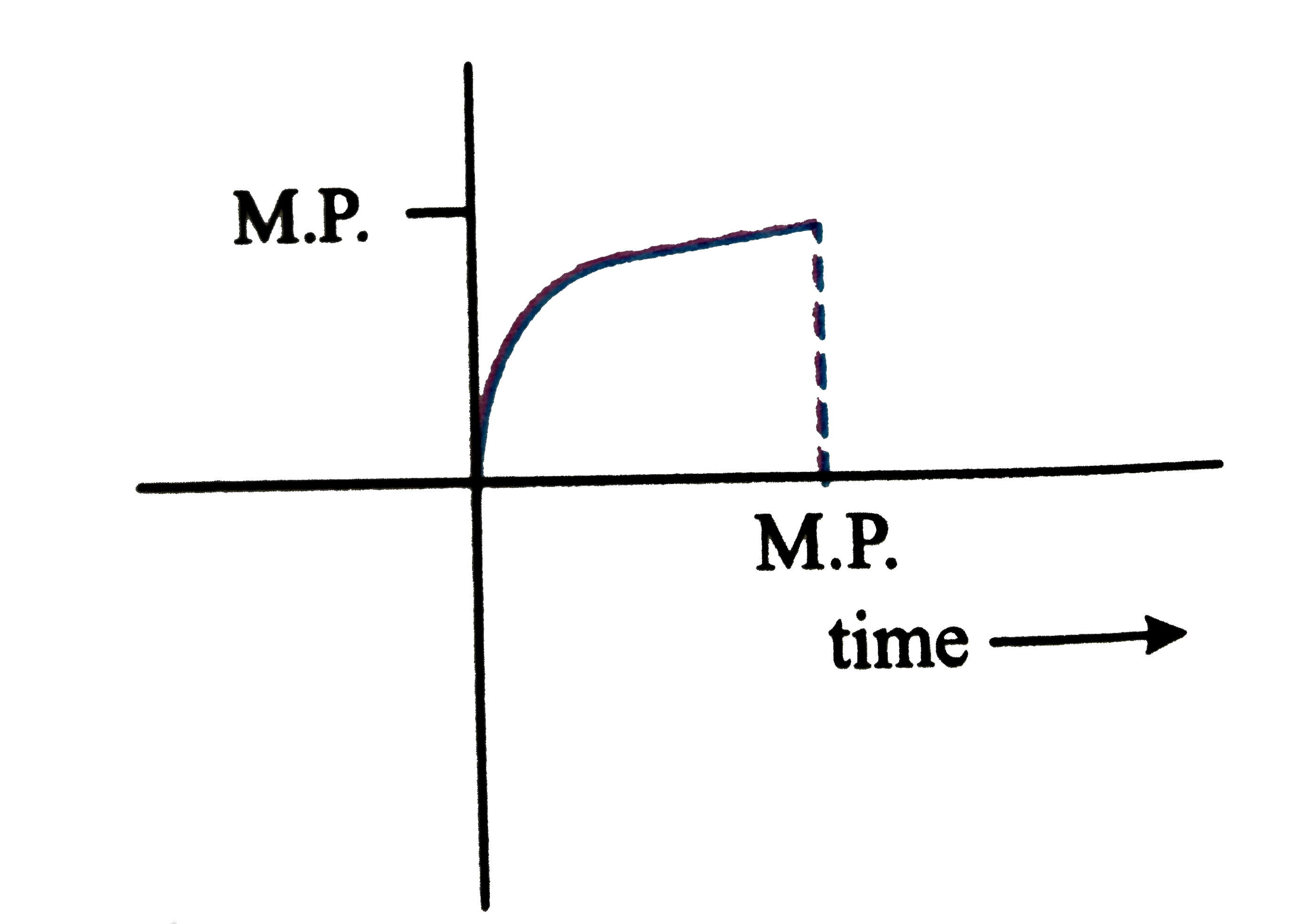
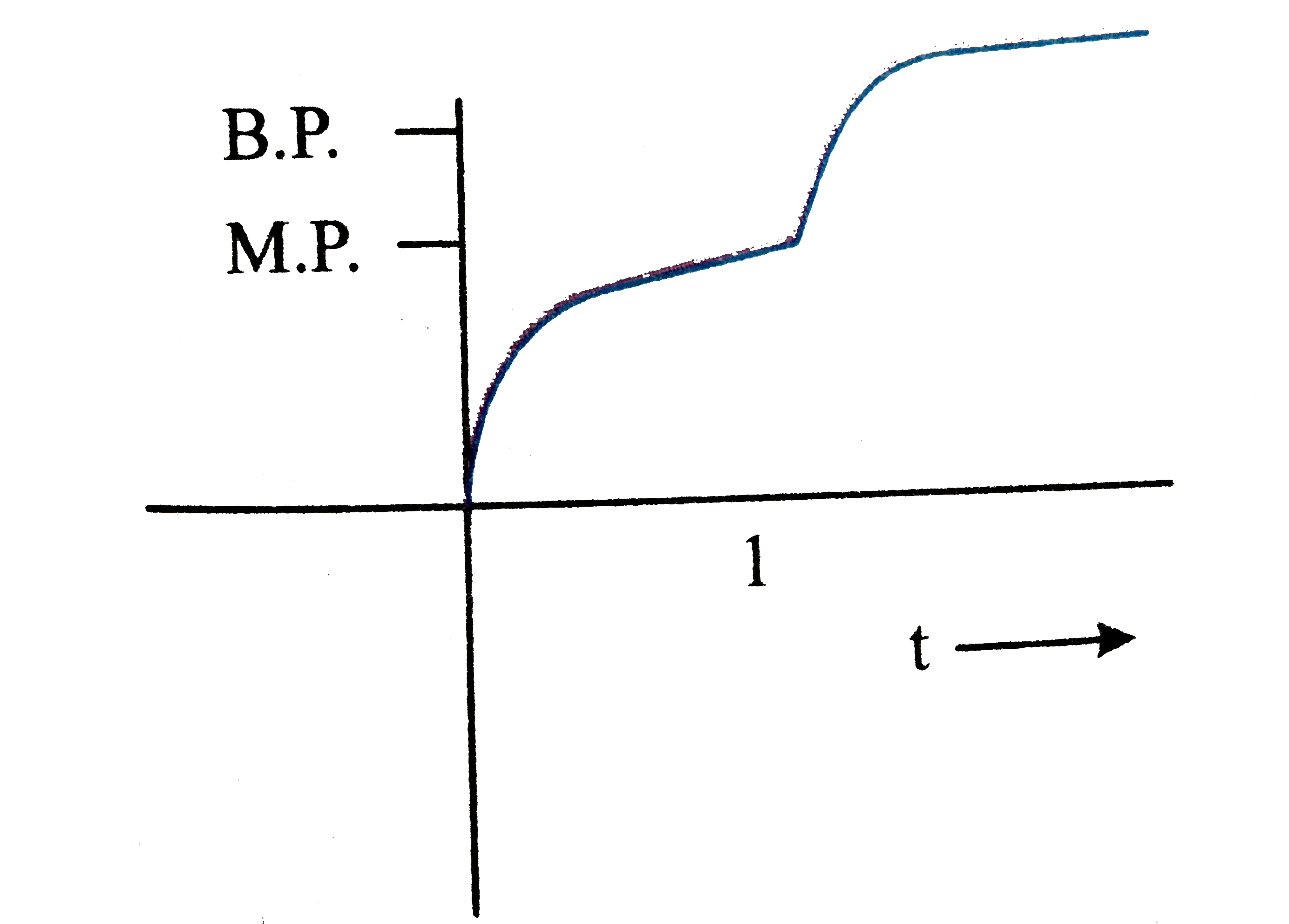
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Exercise
- A 0.50 kg ice cube at -10^(@)C is placed in 3.0 kg of coffce at 20^(@)...
Text Solution
|
- A thermal insulated vessel contains some water at 0^(@)C. The vessel i...
Text Solution
|
- If specific heat capacity of a substance in solid and liquid is propor...
Text Solution
|
- 450 g water at 40^(@)C was kept in a calorimeter of water equivalent 5...
Text Solution
|
- 5 moles of nitrogen gas are enclosed in an adiabatic cylindrical vesse...
Text Solution
|
- When water is boiled at 2 atm pressure the latent heat of vapourizatio...
Text Solution
|
- A vertical cylinder of cross-section area A contains one mole of an id...
Text Solution
|
- A gas is enclosed in a cylinder with a movable frictionless piston. It...
Text Solution
|
- One mole of an ideal monoatomaic gas is taken from A to C along the pa...
Text Solution
|
- Two cylinders are connected by a fixed diathermic partition A and a re...
Text Solution
|
- An ideal monoatomic gas is confined in a horizontal cylinder by a spri...
Text Solution
|
- The molar specific heat of a gas is defined as C=(Dq)/(ndT) Where dQ=h...
Text Solution
|
- The molar specific heat of a gas is defined as C=(Dq)/(ndT) Where dQ=h...
Text Solution
|
- The molar specific heat of a gas is defined as C=(Dq)/(ndT) Where dQ=h...
Text Solution
|
- The system shown in the figure is in equilibrium. The piston is massle...
Text Solution
|
- The system shown in the figure is in equilibrium. The piston is massle...
Text Solution
|
- The system shown in the figure is in equilibrium. The piston is massle...
Text Solution
|
- The rectangular box shown in Fig has partition which can slide without...
Text Solution
|
- The rectangular box shown in Fig has partition which can slide without...
Text Solution
|
- The rectangular box shown in Fig has partition which can slide without...
Text Solution
|