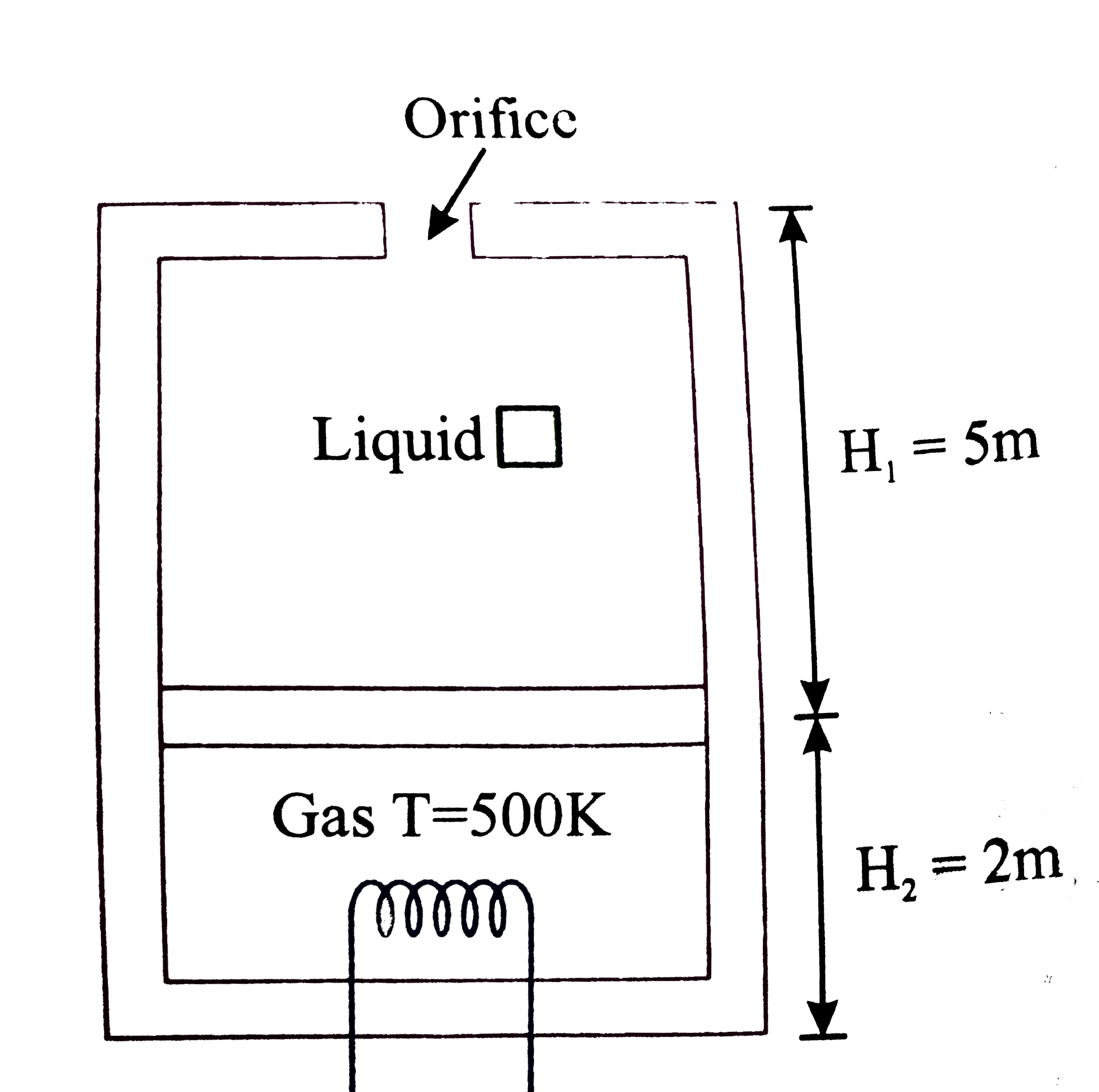
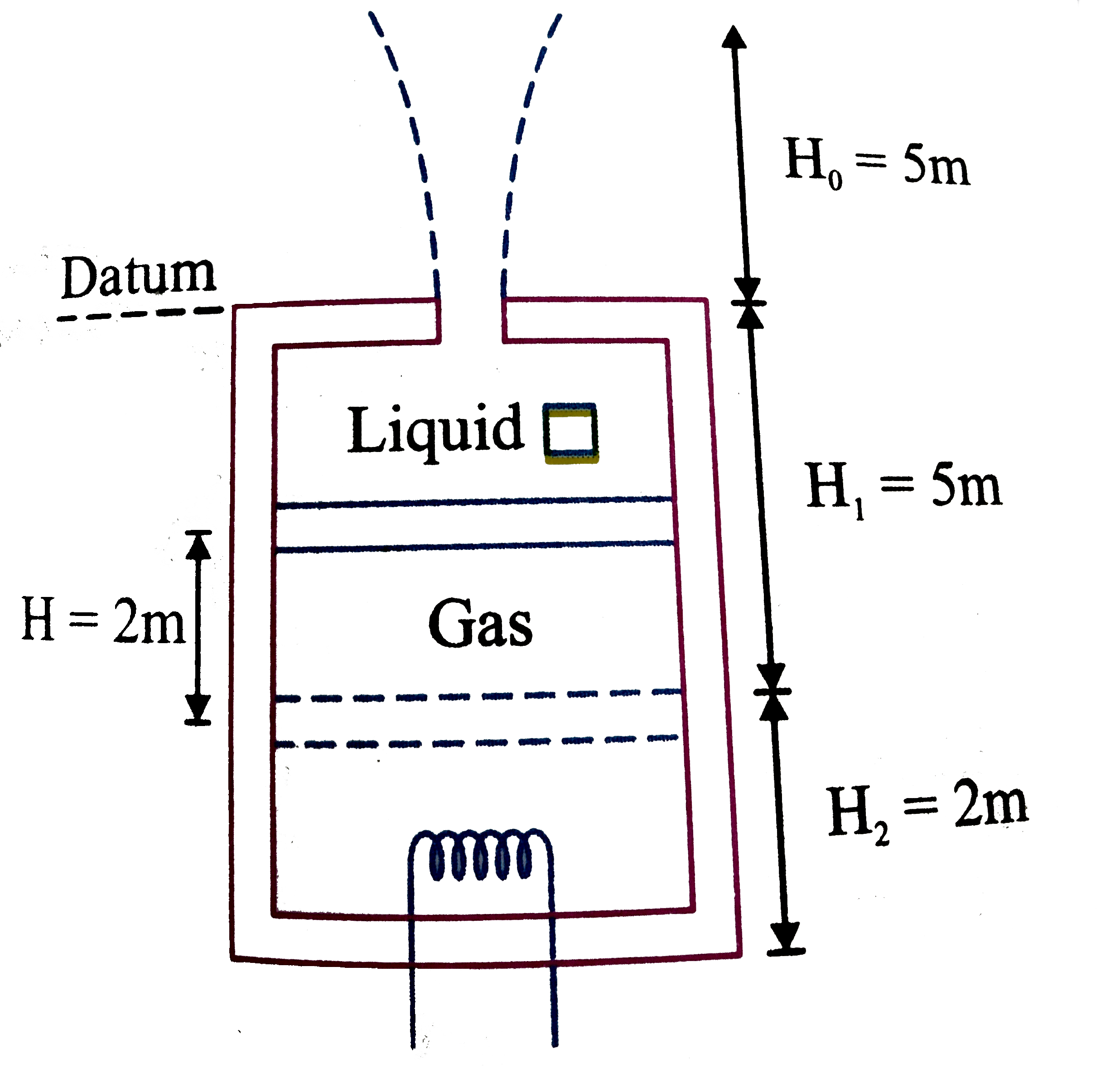
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Exercise
- The molar specific heat of a gas is defined as C=(Dq)/(ndT) Where dQ=h...
Text Solution
|
- The system shown in the figure is in equilibrium. The piston is massle...
Text Solution
|
- The system shown in the figure is in equilibrium. The piston is massle...
Text Solution
|
- The system shown in the figure is in equilibrium. The piston is massle...
Text Solution
|
- The rectangular box shown in Fig has partition which can slide without...
Text Solution
|
- The rectangular box shown in Fig has partition which can slide without...
Text Solution
|
- The rectangular box shown in Fig has partition which can slide without...
Text Solution
|
- An ideal monoatomic gas indergoes a pressure pV(n)=constant. The adiab...
Text Solution
|
- An ideal monoatomic gas indergoes a pressure pV(n)=constant. The adiab...
Text Solution
|
- 2000 mole of an ideal diatmic gas is enclosed in a vertica cylinder fi...
Text Solution
|
- 2000 mole of an ideal diatmic gas is enclosed in a vertica cylinder fi...
Text Solution
|
- 2000 mole of an ideal diatmic gas is enclosed in a vertica cylinder fi...
Text Solution
|
- In Fig., a container is shown to have a movable (without friction) pis...
Text Solution
|
- In Fig., a container is shown to have a movable (without friction) pis...
Text Solution
|
- A container of volume 4V(0) made of a perfectly non- conducting materi...
Text Solution
|
- A container of volume 4V(0) made of a perfectly non- conducting materi...
Text Solution
|
- A container of volume 4V(0) made of a perfectly non- conducting materi...
Text Solution
|
- One mole of an ideal monatomic gas undergoes the process p=alphaT^(1//...
Text Solution
|
- A thermodynamic system is taken from an initial state I with internal ...
Text Solution
|
- A gaseous mixture enclosed in a vessel consists of one gram mole of a ...
Text Solution
|