Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Exercise
- A gaseous mixture enclosed in a vessel consists of one gram mole of a ...
Text Solution
|
- A lead ball at 30^(@)C is dropped from a height h. The ball is heated ...
Text Solution
|
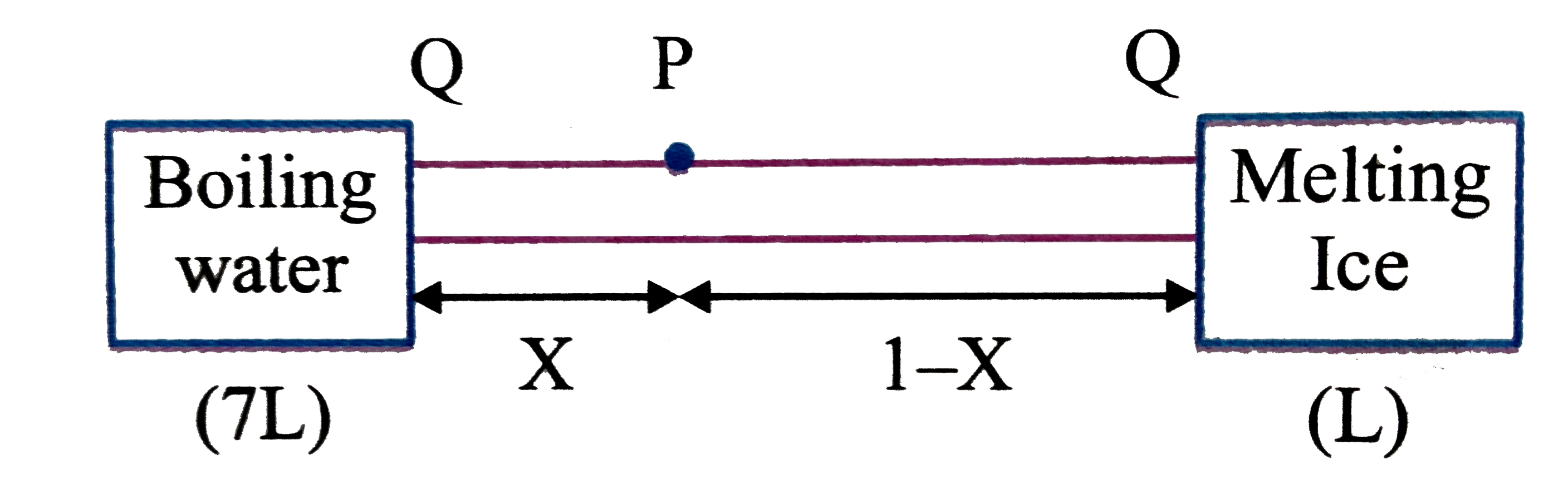
- One mole of a uniform rod of length 1 m is placed in boiling water whi...
Text Solution
|
- One mole of an ideal Po monoatomic gas is taken 4op through a thermo d...
Text Solution
|
- One mole of an ideal gas whose pressure changes with volume as P=alph...
Text Solution
|
- A piston can freely move inside a horizontal cylinder closed from both...
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- A piece of aluminium falls from a height of 200m on a fixed non conduc...
Text Solution
|
- Hail stone fall from certain heigh. If only 2% of the mass of the hail...
Text Solution
|
- From what minimum height a block of ice has to be dropped in order tha...
Text Solution
|
- Two spheres 'A' and 'B' of masses in the rario 1:2 Specific heats in t...
Text Solution
|
- 2kg ice block should be dropped form 'x' km' height to melt completely...
Text Solution
|
- Two metal balls having masses 50g and 100g collides with a target wit...
Text Solution
|
- A brick weighing 4.0 kg is dropped into a 1.0m deep river from a heig...
Text Solution
|
- A man of 60 kg gains 1000 cal of heat by eating 5 mangoes. His efficie...
Text Solution
|
- 2kg of water is converted into steam by bolling at atmosperic pressure...
Text Solution
|
- Find the external workdone by the system in K cal, when 12.5 k cal of ...
Text Solution
|
- Heat of 20 K cal is supplied to the system and 8400 J of external work...
Text Solution
|
- A gas expands from 40 liters to 90 liters at a constant pressure of 8 ...
Text Solution
|
- To a system 300 joules od heat is given and it does 60 joules of work....
Text Solution
|